Association between Thyroid Cancer and Breast Cancer (About 50 Cases)
Iftahy FZ1, El Aziz S1, Chadli A1*
1 Endocrinology, Diabetology and Metabolic Diseases Department, Ibn Rochd University Hospital of Casablanca, Morocco.
1 Neurosciences and Mental Health Laboratory, Morocco.
1 Faculty of Medicine and Pharmacy, University Hassan II, Casablanca, Morocco.
*Corresponding Author
Asma Chadli,
Endocrinology, Diabetology and Metabolic Diseases Department,
Ibn Rochd University Hospital of Casablanca, Morocco.
Tel: +212 6 61092820
E-mail: drachadli@gmail.com
Received: April 02, 2019; Accepted: May 28, 2019; Published: May 31, 2019
Citation: Iftahy FZ, El Aziz S, Chadli A. Association between Thyroid Cancer and Breast Cancer (About 50 Cases). Int J Surg Res. 2019;S1:02:001:1-5. doi: dx.doi.org/10.19070/2379-156X-SI02-01001
Copyright: Asma Chadli© 2019. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Several studies have demonstrated the relationship between breast and thyroid cancer. The aim of the study was to determine factors favoring this association.
Material and Methods: A descriptive retrospective study was conducted in our entity, including 50 patients followed for thyroid and breast carcinoma between 1986-2018 among all thyroid differentiated carcinomas (680 patients).
Results: The prevalence was 7.35%. Mean age was 42 ± 6.5 years. Breast cancer preceded the discovery of thyroid cancer in half of the cases.45% of subjects received external beam radiotherapy. Predictor factors of this association were female patients who had the first neoplasia at a young age (<45 years), family history of neoplasia and prior external radiotherapy.
Conclusion: The results of our study support the hypothesis of this, so several authors recommend greater vigilance regarding early detection of thyroid cancer in patients treated for breast cancer.
2.Background
3.Patients and Methods
4.Results
5.Discussion
6.Conclusion
7.References
Keywords
Thyroid Carcinoma; Breast Carcinoma; Radiotherapy.
Background
The overall incidence of thyroid cancer has increased dramatically in the world for several decades [1, 2]. Thyroid carcinoma is estimated to be the third most common cancer in women of all ages and the second most common cancer in women under 45 years with good prognosis and 95% survival at 5-10 years [3, 4].
Survivors of thyroid cancer have a high risk of developing a second primary malignancy compared to the general population, including breast cancer with a cumulative incidence of 36% at 7-10 years after diagnosis of thyroid cancer [1-5].
The association between the incidence of these two types of cancer was previously found and studied in several cohorts [6, 7].
Several hypotheses have been developed to explain this association. This could be related to the initial tumor treatment, particularly radiotherapy, or to genetic and/or hormonal factors, but no definitive relationship has been established [8, 9].
Our work had the primary objective of assessing the prevalence of breast cancer in patients treated for thyroid carcinomain our entity and to determine the group of patients at high risk of developing a syndrome of multiple primary cancers associating both cancers through the study of the different epidemiological, histological and therapeutic aspects of our patients.
Patients and Methods
We realized a descriptive retrospective study over a period of 32 years including all patients followed for thyroid carcinoma in Endocrinology and Diabetology Department of the University Hospital Center Ibn ROCHD of Casablanca) between 1986 and 2018 (680 patients).
Among these, 50 patients had also breast cancer discovered in routine screening by a clinical examination completed with ultrasound and X-ray mammogram and confirmed histologically after surgery. We excluded all non-adherent patients and those lost of sight.
Data were collected through the patient records study relating to age, personal and family history of neoplasia, various treatments received: surgery, radiotherapy and radioiodine therapy, free interval between the breast and thyroid cancer diagnosis, the histological type of both cancers and other histological features of thyroid carcinoma such as tumor size, thyroid capsular spread, multifocality and finally patients evolution. While the main recommendations used the age of 45 as a threshold for dividing patients [10, 11], we therefore divided our patients into 2 groups: The younger group (age <45 years) and the older one (age ≥ 45 years).
Thyroid cancers have been classified according to the WHO Classification of Tumours of Endocrine Organs (4th edition) [12].
Statistical analysis was performed using SPSS software statistics 25 (chi squared test). The difference was considered significant when a value of p was <0.05.
Results
Among 680 patients followed for thyroid carcinoma, 50 patients had breast cancer associated with a prevalence of 7.35%.
Mean age of the patients was 42 ± 6.5 years old with age range between 36 and 70 years. A family history of hormonal cancers was found in 50% of cases. Breast cancer preceded the discovery of thyroid cancer in half of cases with an average delay of 5.5 years (6 months and 20 years). Table 1 summarizes the different clinicopathological features of these patients.
Regarding the treatment of thyroid carcinoma, all patients had undergone a total thyroidectomy associated with radioiodine therapy whose main indication was multifocality as well as the capsular spread.
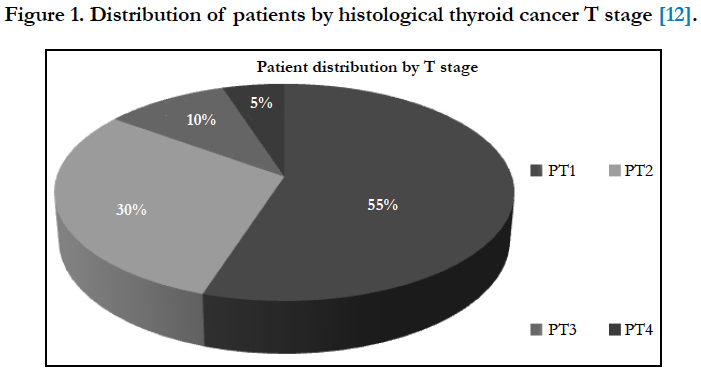
Papillary carcinoma was the predominant histological type in 96% of cases with an average tumor size of 14 mm and predominance of the PT1 stage in 55% of cases according to the T stage as demonstrated in Figure 1.
Regarding treatment of breast cancer, an unilateral mastectomy associated with lymph node dissection was performed in 72% of cases supplemented by chemotherapy and hormone therapy. Adjuvant treatment with external radiotherapy was performed in 45% of patients. Invasive ductal carcinoma was the predominant histological type in 93% of cases followed by ductal carcinoma in situ in 7% of cases. Lymph node metastases were found in 20% of patients and one patient had distant metastases at the time of diagnosis.
The results of the comparative study between the two groups of patients (the first group having a thyroid carcinoma isolated and the second one having both neoplasms) were reported in Table 2. Patients with the both primary cancers associated were younger (P <0.01), had a family history of neoplasia in 50% of cases with a significant difference (p = 0.02) including 10 cases of familial breast cancer, 6 cases of endometrial cancer, 5 cases of ovarian cancer and 4 cases of colorectal cancer. Thyroid carcinoma was smaller with more pronounced multifocality (p <0.01). Neoadjuvant radiotherapy treatment was a factor supporting this association (p <0.02).
Table 2. Comparison of Clinicopathological Characteristics in the 2 groups of patients.
Group 1 : thyroid cancer versus Group 2 : thyroid and breast cancer.
Moreover, there was no significant difference between the two groups in relation to the histological type nor the adjuvant treatment, especially the radioiodine therapy.
Recurrences were comparable between the two groups without significant difference. One death was noted in a 70-year-old patient followed during 2 years for papillary carcinoma (pT2) associated with late stage of breast cancer with distant metastases
Discussion
In recent years, many studies have focused on the relationship between the coexistence of breast cancer and thyroid carcinoma. Several of them have shown that the incidence of this association is clearly increasing compared to the general population [5, 13]. Both studies Consorti et al., [14] as well as Fang yu et al., [9] revealed that the prevalence of breast cancer was significantly higher in patients with thyroid cancer. In our study, the prevalence was higher (7.35%) compared to other studies.
Several hypotheses have been established to explain this association with genetic origin as the main factor. Lynch syndrome is characterized by the presence of mutations in the genes responsible for DNA repair, which can lead to an increased risk of malignancies, including colorectal cancer, endometrial carcinoma, ovarian cancer, uterus cancer, gastric cancer, and small bowel cancer [1, 15]. Recently, studies have reported the presence of a common mutation of the same DNA repair gene between breast and thyroid cancer, so they have been considered an expansion of the Lynch syndrome tumor spectrum [16, 17].
The role of clinicopathological features in increasing the incidence and prevalence of this association remains controversial. Fang Yu et al., [9] concluded in his study that patients treated for breast cancer associated thyroid cancer were younger (age <45 years), with a tumor size <15mm and with less locoregional metastases compared to those with isolated thyroid cancer. These results were also found by the study of H. Kuo [18]. This is consistent with the results of our study who found that patients with both associated cancers were younger (<45 years), had a family history of cancer, and had a smaller tumor size than those with isolated thyroid cancer.
The most histological type of thyroid cancer commonly associated with breast cancer is papillary cancer in 85.9% followed by follicular carcinoma in 11% of cases [19]. This is consistent with the results of our study, which found that papillary carcinoma is also the most common histological type in 96% of cases.
The contribution of chemotherapy and radiotherapy in the genesis of a secondary cancer remains controversial. It has been reported that positron emission tomography (CT) and chest CT scans, which are the two commonly used tests in breast cancer follow-up, may contribute to an increase in the rate of thyroid cancer [1, 20].
However, Sun et al., [21] suggests that radiotherapy does not increase the risk of thyroid cancer in women with breast cancer. In our study neoadjuvant radiotherapy was a factor supporting this association with a significant result (p <0.01). Recently, it has been suggested that chemotherapy treatment, particularly with docetaxel and cyclophosphamide, may contribute to the genesis of secondary thyroid cancer. Moreover, no large study has confirmed this hypothesis [9]. In our study, chemotherapy type could not be specified due to lack of data in patients with prior breast cancer which constitutes a bias.
The time to onset of secondary cancer versus primary cancer varies by study, H. Kuo et al., [18] reported in his study that breast cancer was diagnosed within an average of 5 years after diagnosis of thyroid cancer. This is consistent with our results reporting an average interval of 5.5 years (6 months-20 years).
Our work also has some limitations including the study type, given its retrospective character which is the main selection bias, and given the lack of some clinical and therapeutic patients data such as the doses of external beam radiotherapy nor the duration of the treatment nor the interval between the radiotherapy and the genesis of the thyroid cancer. Selection bias may also account for the higher rates of neoadjuvant radiation in the combined group. Also tumor size discrepancy may be due to surveillance bias, depending on how the thyroid cancer was diagnosed.
Conclusion
Our study results support the hypothesis of the presence of a relationship between the breast cancer occurrence in patients followed for thyroid cancer.
Waiting for confirmation of these preliminary results, several authors recommend greater vigilance regarding the early detection of breast cancer in patients followed for thyroid cancer, mainly in patients who had the first cancer at young age (<45 years old) with a family history of neoplasia and a notion of prior external radiotherapy.
References
- Dong L, Lu J, Zhao B, Wang W, Zhao Y. Review of the possible association between thyroid and breast carcinoma. World J Surg Oncol. 2018 Jul 5;16(1):130. doi: 10.1186/s12957-018-1436-0. PubMed PMID: 29976206.
- Kilfoy BA, Devesa SS, Ward MH, Zhang Y, Rosenberg PS, Holford TR, et al. Gender is an age-specific effect modifier for papillary cancers of the thyroid gland. Cancer Epidemiol Biomarkers Prev. 2009 Apr;18(4):1092-100. doi: 10.1158/1055-9965.EPI-08-0976. PubMed PMID: 19293311.
- Aschebrook-Kilfoy B, Kaplan EL, Chiu BC, Angelos P, Grogan RH. The acceleration in papillary thyroid cancer incidence rates is similar among racial and ethnic groups in the United States. Ann Surg Oncol. 2013 Aug;20(8):2746-53. doi: 10.1245/s10434-013-2892-y. PubMed PMID: 23504142.
- Garner CN, Ganetzky R, Brainard J, Hammel JP, Berber E, Siperstein AE, et al. Increased prevalence of breast cancer among patients with thyroid and parathyroid disease. Surgery. 2007 Dec;142(6):806-13. PubMed PMID: 18063060.
- Ronckers CM, McCarron P, Ron E. Thyroid cancer and multiple primary tumors in the SEER cancer registries. Int J Cancer. 2005 Nov 1;117(2):281-8. PubMed PMID: 15880372.
- Khang AR, Cho SW, Choi HS, Ahn HY, Yoo WS, Kim KW, et al. The risk of second primary malignancy is increased in differentiated thyroid cancer patients with a cumulative (131)I dose over 37 GBq. Clin Endocrinol (Oxf). 2015 Jul;83(1):117-23. doi: 10.1111/cen.12581. PubMed PMID: 25115234.
- Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014 Feb;138(2):241-56. doi: 10.5858/arpa.2013-0953-SA. PubMed PMID: 24099077.
- Kumar A, Klinge CM, Goldstein RE. Estradiol-induced proliferation of papillary and follicular thyroid cancer cells is mediated by estrogen receptors alpha and beta. Int J Oncol. 2010 May;36(5):1067-80. PubMed PMID: 20372779.
- Yu F, Ma J, Huo K, Li P. Association between breast cancer and thyroid cancer: a descriptive study. Transl Cancer Res. 2017 Jul 4;6(2):393-401.
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016 Jan;26(1):1-133. doi: 10.1089/thy.2015.0020. PubMed PMID: 26462967.
- Mitchell AL, Gandhi A, Scott-Coombes D, Perros P. Management of thyroid cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016 May;130(S2):S150-S160. PubMed PMID: 27841128.
- Lloyd RV, Osamura RY, Kloppel G, Rosai J. WHO Classification of Tumours of Endocrine Organs. 4th edn. IARC: Lyon, France; 2017.
- Oeffinger KC, Baxi SS, Novetsky Friedman D, Moskowitz CS. Oeffinger KC1, Baxi SS2, Novetsky Friedman D3, Moskowitz CS4. Semin Oncol. 2013 Dec;40(6):676-89. doi: 10.1053/j.seminoncol.2013.09.012. PubMed PMID: 24331190.
- Consorti F, Di Tanna G, Milazzo F, Antonaci A. Nulliparity enhances the risk of second primary malignancy of the breast in a cohort of women treated for thyroid cancer. World J Surg Oncol. 2011 Aug 12;9:88. doi: 10.1186/1477-7819-9-88. PubMed PMID: 21835042.
- Burn J, Mathers J, Bishop DT. Lynch syndrome: history, causes, diagnosis, treatment and prevention (CAPP2 trial). Dig Dis. 2012;30 Suppl 2:39-47. doi: 10.1159/000341892. PubMed PMID: 23207931.
- Harkness EF, Barrow E, Newton K, Green K, Clancy T, Lalloo F, et al. Lynch syndrome caused by MLH1 mutations is associated with an increased risk of breast cancer: a cohort study. J Med Genet. 2015 Aug;52(8):553-6. doi: 10.1136/jmedgenet-2015-103216. PubMed PMID: 26101330.
- Ford JM. Is breast cancer a part of Lynch syndrome?. Breast Cancer Res. 2012 Aug 22;14(4):110. doi: 10.1186/bcr3241. PubMed PMID: 22913763.
- Kuo JH, Chabot JA, Lee JA. Breast cancer in thyroid cancer survivors: An analysis of the Surveillance, Epidemiology, and End Results-9 database. Surgery. 2016 Jan;159(1):23-9. doi: 10.1016/j.surg.2015.10.009. PubMed PMID: 26522696.
- Lee J, Park S, Kim S, Kim J, Ryu J, Park HS, et al. Characteristics and Survival of Breast Cancer Patients with Multiple Synchronous or Metachronous Primary Cancers. Yonsei Med J. 2015 Sep;56(5):1213-20. doi: 10.3349/ymj.2015.56.5.1213. PubMed PMID: 26256962.
- Pelizzo MR, Pennelli G, Zane M, Galuppini F, Colletti PM, Merante Boschin I, et al. Papillary thyroid carcinoma (PTC) in Lynch syndrome: Report of two cases and discussion on Lynch syndrome behaviour and genetics. Biomed Pharmacother. 2015 Aug;74:9-16. doi: 10.1016/j.biopha. 2015.06.008. PubMed PMID: 26349957.
- Sun LM, Lin CL, Liang JA, Huang WS, Kao CH. Radiotherapy did not increase thyroid cancer risk among women with breast cancer: A nationwide population-based cohort study. Int J Cancer. 2015 Dec 15;137(12):2896-903. doi: 10.1002/ijc.29667. PubMed PMID: 26135015.