A Case of Synchronous Renal and Splenic Artery Embolism in a Patient with Atrial Fibrillation
Maria Tsachiridi
General Surgeon, Upper GI Fellow, Royal Derby Hospital, Thornhill Road Derby, UK.
*Corresponding Author
Maria Tsachiridi,
General Surgeon, Upper GI Fellow, Royal Derby Hospital,
13 Thornhill Road Derby, DE22 3LX, UK.
Tel: +447756650699
E-mail: tsachimaria@yahoo.com
Received: November 23, 2015; Accepted: January 25, 2016; Published: January 27, 2016
Citation: Maria Tsachiridi (2016) A Case of Synchronous Renal and Splenic Artery Embolism in a Patient with Atrial Fibrillation. Int J Surg Res. 3(1), 39-42.doi: dx.doi.org/10.19070/2379-156X-160008
Copyright: Maria Tsachiridi© 2016. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: Renal and splenic artery embolism are rare conditions and even more rarely they present simultaneously in the same patient.
Case Report: We present a case of patient with renal and splenic artery embolism, their presentation, diagnosis, treatment and outcome along with the results of two year follow up.
Discussion: As no clinical studies have been performed to define diagnostic and treatment pathways and guidelines, clinicians have to rely on literature mostly comprised of case reports to lead them. The imaging options described are that of CT scan followed by MRI, Ultrasound and other invasive methods such as Angiogram. Two treatment options have been proposed by the authors, invasive (angiogram and thrombolysis), which are reserved for early diagnosed cases, and conservative for cases diagnosed after 48 hours of presentation.
Conclusion: As renal and splenic artery embolism are often misdiagnosed, doctors need to maintain high grade of suspicion to be able to correctly recognize and treat these patients, especially the very rare cases of bilateral renal embolism where the renal function compromise can be preventable and life saving.
2.Introduction
3.Case Report
3.1 Presentation
3.2 History and Medications
3.3 Physical Examination
3.4 Laboratory Findings
3.5 Imaging
3.6 Other Specialty Advice
3.7 Treatment
3.8 Outcome
3.9 Follow Up
4.Discussion
5.Conclusion
6.References
Keywords
Renal Artery Embolism; Splenic Artery Embolism; Atrial Fibrillation; Renal Infarction; Splenic Infarction.
Introduction
Renal artery embolism is a very rare condition often misdiagnosed by the majority of primary physicians as its presentation resembles a variety of medical conditions [1], even more rare is its combination with other types of embolism like splenic infarction, pulmonary embolism, mesenteric or hepatic artery embolism [2].
Case Report
We present a case of renal and splenic artery embolism on the background of Atrial Fibrillation without an adequate therapy.
A 83 years old female presented to an emergency department with a history of sudden onset of acute sharp right flank pain. The pain started two days before, driving her to attend another hospital, from which she was discharged with the diagnosis of renal colic. Now she described the pain as persisting dull pain in the right flank and epigastrium.
The patient had a history of Atrial Fibrillation (AF) and Hypertension. Her medications included: Valsartan 80 mg once daily (OD), Carvedilol 3,125 mg OD, Aspirine 100 mg OD. She could not recall how long ago she was diagnosed with AF but she knew from her doctor that she had arrhythmia, however she was not taking any anticoagulants, apart from Aspirin. The patient denied previous thromboembolic episodes.
Physical exam showed no peritoneal signs, rebound or guarding but a mild tenderness, in the right upper quadrant.
Heart rate 100bpm, irregular, blood pressure 140/80 mmHg, electrocardiogram (ECG) demonstrated Atrial Fibrillation.
Laboratory results showed significant leukocytosis, white cell count (WBC): 26 200, Neutrophils (NE%): 87.9%, Hematocrit: 47.8%, Platelet count (PLT): 173 000, altered renal function (Creatinine:1.4 mg/dl), mildly elevated transaminases: Aspartate aminotransferase (SGOT/AST): 71 U/L, Alanine aminotransferase (SGPT/ALT): 67 U/L, elevated Lactate dehydrogenase (LDH): 1871 IU/L, Creatine phosphokinase (CPK): 210 mU/ml and Troponine: 0,07 ng/ml, D-Dimers: 2,66 μg/L, Fibrinogen: 665 mg/dL, normal coagulation profile: Prothrombine time (PT): 12 sec, Activated Partial Tromboplastine time (APTT): 35 sec, International Normalization Ratio (INR): 1.0.
The urinalysis revealed microscopic haematuria Red cells (RBC): 70-80 per optical field, Haemoglobin (HGB) ++++ and albuminuria, Albumin (Alb) +++.
Ultrasound scan (US) had no abnormal findings.
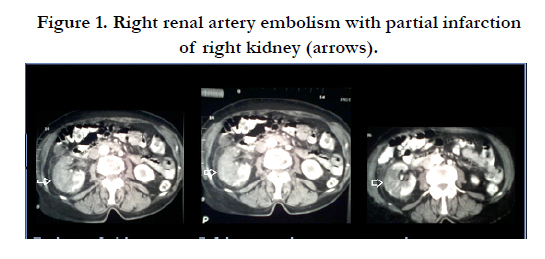
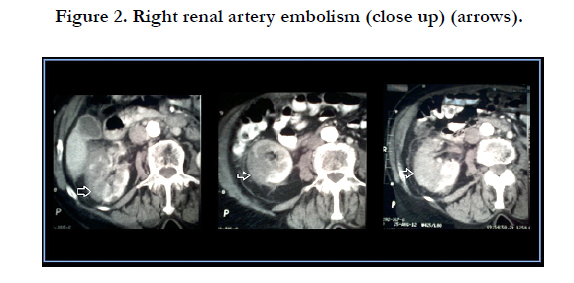
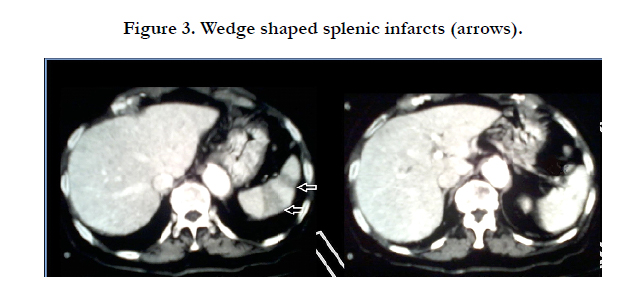
Arterial phase shows normal enhancement of the trunk of the right renal artery with lack of opacification of the arterial branches of the hilum of the right kidney. Extended hypoattenuated areas in the upper pole and the middle of the right kidney, and preserved blood flow to the cortex of the lower pole. Small fluid collection around the right kidney and perirenal fat stranding are also present (Figure 1 and 2). Extended wedge shaped hypodense areas of infarction in the spleen (Figure 3).
The Urology and Vascular Surgery advice was sought and they suggested conservative treatment and anticoagulation as the initial presentation preceded more than 48 hours.
The patient was initially treated with broad spectrum antibiotics (Tazobactam - Piperacillin) as she was considered to be septic. The differential diagnosis included urinary tract infection, pyelonephritis and cholecystitis among others as the most common causes of right upper quadrant and flank pain that could possibly be the cause of septic picture. After the CT scan was acquired and the embolism was diagnosed the antibiotics were discontinued and low molecular weight heparin (LMWH), enoxaparin, and warfarin were initiated. When the target International Normalization Ratio (INR) rate of 2.5-3 was achieved, the enoxaparin was discontinued.
The creatinine values rose up to peak of 2.0 mg/dL during the hospital stay and then dropped gradually. The white blood count was normalized within 5 days as well as the other blood indices. The patient was discharged after 10 days.
After 2 years of follow up the patient is in good general condition. On new US scan the right kidney was reduced in size, 5.8 cm, with abnormal edge and cortical refinement at the upper pole. The cortex of the lower pole was preserved. The laboratory exams showed creatinine levels of 1.2 mg/dL (normal range 0.5- 1.0 mg/dL), LDH: 261 IU/L (normal range 135-225 IU/L). On electrocardiogram Atrial Fibrillation was steadily present. The patient continues taking warfarin. During this period of time she did not present any thromboembolic episode except for mild transient ischaemic cerebral episode without any radiological findings, that did not leave any permanent dysfunction.
Discussion
The diagnosis of the acute renal embolism is usually missed, due to lack of awareness, low level of suspicion and non-specific clinical presentation. The incidence of the disease has been reported as 0.007% of admissions or 6.1 patients per million [3], and 2% of all peripheral arterial thromboembolism events due to AF [4]. Among the risk factors and causes of thromboembolism are: AF, previous embolism (stroke [4]), mitral stenosis, ischaemic heart disease, anticoagulation therapy and abnormal coagulation profile [3], cardiomyopathy and valvular disease [5], myocardial infarction and rheumatic mitral stenosis [6], thrombosis complicating endovascular intervention [7] as well as oral contraceptives [8], paradoxical embolism [2], postoperative [9] and idiopathic renal embolism [10]. With the AF having the most significant role and being the most investigated.
The typical clinical presentation is of acute onset of sudden, sharp abdominal, flank, epigastric or back pain [1, 3, 5, 11, 12]. The pain is constant without relieving position, nausea, vomiting and fever can be present. Blood pressure can be elevated due to a renin mediated mechanism [6], but in the majority of cases it is normal or within the normal for the patient rates. The mean age at presentation is 65, 7 years [1]. Very rare is a bilateral embolism, around 10% [1] and it also is followed by more severe clinical presentation [13].
The differential diagnosis includes causes of acute surgical abdomen, such as appendicitis, diverticulitis, ruptured aortic aneurism, intestinal obstruction or perforation, incarcerated hernia, testicular or ovarian torsion [1], others such as mesenteric ischaemia, nephrolithiasis and renal colic, pyelonephritis and acute cholecystitis [12]. The diagnosis is rarely made on admission and is often delayed to two or more days [1, 3, 12-14].
Typical laboratory findings include microscopic haematuria [1, 3, 5, 13], although not always present on admission but one or two days after the onset of symptoms [14], mild proteinuria [12-14], creatinine elevation that usually reaches a peak of 2-3, 5 mg/dl [1, 3, 12, 15] and then returns to normal rates, significant elevation of LDH is observed in all the cases. Lactate hydrogenase is present in renal tissue and its elevation indicates renal injury [1], therefore studies should be performed to show whether or not it is correlated to the severity of the infarct and could be used as a strong laboratory index of the renal embolism. Also mild elevation of transaminases and C reactive protein can be observed [1, 3, 5, 12 - 15]. High white blood cell count has been associated to thromboembolism by some authors but investigated mostly in Pulmonary Embolism [25, 26]. Though most articles describing cases of renal and splenic embolism mention elevated white cell count, more research is required to determine whether this finding can be directly correlated to thromboembolic disease or be used to predict severity or outcome.
Despite the lack of systematic research all the authors seem to agree that spiral CT is an imaging modality of choice [1], although some of them suggest obtaining an unenhanced CT scan to rule out nephrolithiasis and thus renal colic and continue with contrast-enhanced CT scan to identify a possible infarction [3, 5]. Common findings are hypoattenuated area (wedge-shaped) corresponding to a non-functional renal tissue and a cortical rim sign due to collateral blood supply [1, 5] as well as peripheral stranding with thickening of Gerota’s facia [3]. Angiogram is the best method of identifying renal infarction but being an invasive method is preserved for the patients with a negative CT scan but at high risk of thromboembolic events and those whose diagnosis is established, as a definitive treatment [1, 12, 15]. Other methods include Magnetic Resonance Imaging (MRI), MRI angiogram (MRA), renal isotope scan and ultrasound but these are not widely used especially when the use of CT scan is facilitated.
Though a well-designed prospective study comparing the different treatment options for renal infarction does not exist, there are two approaches generally accepted by most of the authors. The first is the initiation of IV heparin bolus and heparin infusion, though everyone uses their own dosage protocol, followed by oral warfarin to the target INR of 2-3 [3, 5, 11, 14]. The other option proposed is an angiogram followed by selective intra vascular infusion of TPA (tissue plasminogen agent) [15], urokinase [12], thrombolysis and stenting [13] and others [7, 16]. This method seems to be restricted by the belief that the ischemic tolerance of the kidney do not exceed 180 minutes [7], however many cases of revascularization beyond this time frame have been reported to have good results [13]. Either of these therapeutic methods seem to have good results with only rare mild renal function decrease and only few cases of end stage renal disease needing haemodialysis reported [3]. Regarding the absence of guidelines or other clinical evidence strongly supporting other approach, the common sensation is to treat renal embolism with anticoagulants, leaving invasive - angiogram, angioplasty - treatment for selected patients, such as those suffering bilateral embolism, unilateral with a single kidney, or an embolism causing severe renal dysfunction and hypertension.
Those patients who develop hypertension due to renal infarction could benefit from angiotensin - converting enzyme inhibitors and angiotensin receptor blockers based on the knowledge that this type of hypertension develops due to increase of renin [17].
The anticoagulation therapy should be continued for life especially in patients with AF and given that a previous thromboembolic episode is an independent risk factor for thromboembolism [3]. Splenic infarction and its clinical features were described by William Osler in 1901 [18], however it rarely presents in the typical way and the clinical presentation of it in modern days is very different from the classical teaching. The typical patient complains of abdominal pain and only the half of patients localize it to the left upper quadrant, abdominal tenderness can be found and splenomegaly is noted in one third of patients in one study [19]. In another study the most common symptom was either abdominal or left flank pain (80% of episodes), while the most common sign was upper left quadrant tenderness (35% of episodes) [20].
The most frequent laboratory findings are the LDH elevation, which occurs in most of the patients, and Alkaline Phosphatase (ALP) and transaminase alterations that are present in only few [19].
The factors that are believed to provoke splenic infarction are bacterial endocarditis, sickle cell anemia, hypercoagulable state, malignancies including haematologic ones, intracardial thrombus due to AF or myocardial infarction among others [19, 21, 22].
Once again many imaging methods have been used to identify a splenic artery embolism and splenic infarction with the CT scan having a predominant role in establishing the definitive diagnosis. Contrast studies should be performed during the appropriate delay (50 sec) when most spleens will be in the uniform phase of enhancement, due to the dual blood supply of the spleen, so that the normal early archiform pattern of arterial splenic enhancement does not mask lesions or create pseudolesions [23]. These postcontrast scans clearly depict the classic segmental, wedge-shaped, low-attenuation defect [24]. Ultrasonographic scanning was found to be diagnostically useful in only 18% of patients [20]. MRI, preferably with intravenous gadolinium contrast, is another useful modality that clearly identifies infarcted splenic parenchyma.
The conservative treatment is the one usually followed (analgesia, antibiotics) with the surgical splenectomy being preserved for the cases with multiple splenic abscesses. Because of the risk of fatal, overwhelming post-splenectomy sepsis, splenic preservation is preferable whenever possible.
The prognosis varies with the underlying disease process responsible for splenic infarction.
Conclusion
The renal and splenic embolisms are rare medical entities. And even rarer is the finding of both these conditions in one patient. Although a complication of a very common disease such as Atrial Fibrillation, both are very often misdiagnosed. Considering the potentially severe complications, such as renal failure and splenic abscess, a high level of suspicion is required for adequate diagnosis and treatment of these conditions. As the existing literature lacks well-designed prospective studies and guidelines for imaging and therapy, the doctors tend to act as aggressively as they consider and with the means they possess. Both the conservative and the invasive approach have good results, with no evidence existing of the requirement of surgical intervention.
References
- Lopez VM, Glauser J (2010) A case of renal artery thrombosis with renal infarction. J Emerg Trauma Shock 3(3): 302.
- Turedi S, Gunduz A, Eroglu O, Hos G, Durmus I, et al. (2007) Paradoxical embolism involving 4 organ systems (pulmonary, renal, splenic, and hepatic artery). Am J Emerg Med 25(6): 737e1-3.
- Korzets Z, Plotkin E, Bernheim J, Zissin R (2002) The clinical spectrum of acute renal infarction. Isr Med Assoc J 4(10): 781-784.
- Frost L, Engholm G, Johnsen S, Moller H, Henneberg EW, et al. (2001) Incident thromboembolism in the aorta and the renal, mesenteric, pelvic, and extremity arteries after discharge from the hospital with a diagnosis of atrial fibrillation. Arch Intern Med 161(2): 272-276.
- Kansal S, Feldman M, Cooksey S, Patel S (2008) Renal artery embolism: a case report and review. J Gen Intern Med 23(5): 644-647.
- Lessman RK, Johnson SF, Coburn JW, Kaufman JJ (1978) Renal artery embolism: clinical features and long-term follow-up of 17 cases. Ann Intern Med 89(4): 477-482.
- Blum U, Billmann P, Krause T, Gabelmann A, Keller E, et al. (1993) Effect of local low-dose thrombolysis on clinical outcome in acute embolic renal artery occlusion. Radiology 189(2): 549-554.
- Bhargava A, Chopra A, Bernabela L, Chopra T (2013) Oral contraceptive causing renal artery thrombosis. BMJ Case Rep.
- Inaba A, Karim M (2014) Postoperative renal artery thrombosis. Clin Exp Nephrol 18(4): 676-677.
- Bolderman R, Oyen R, Verrijcken A, Knockaert D, Vanderschueren S (2006) Idiopathic renal infarction. Am J Med 119(4): 356e9-12.
- Akdur O, Durukan P, Ozkan S, Ikizceli I, Avsarogullari L, et al. (2008) Renal artery embolism in a patient with vague abdominal pain. Ann Acad Med Singapore 37(5): 437.
- Cheng KL, Tseng SS, Tarng DC (2003) Acute renal failure caused by unilateral renal artery thromboembolism. Nephrol Dial Transplant 18(4): 833-835.
- Uta H, Michael K, Hermann P, Martin H, Eckhart B (2008) A rare case of acute renal failure--acute bilateral renal artery embolism. Nephrol Dial Transplant 23(6): 2095-2097.
- Yoshida T, Ikehara N, Miyabe H, Sakata S, Yajima K, et al. (2004) Two cases with renal infarction diagnosed in the early course using contrast-enhanced CT. Hypertens Res 27(7): 523-526.
- Baydar O, Baskurt M, Coskun U, Ersanli M (2013) A case of renal artery embolism treated by selective intra-arterial infusion of tissue plasminogen activator. Turk Kardiyol Dern Ars 41(6): 534-536.
- Wang J, Zhang Y, Sun YM, Zhou Y (2013) Successful catheter aspiration and local low-dose thrombolysis in an acute renal artery embolism. Cardiovasc Revasc Med 14(5): 302-304.
- Paris B, Bobrie G, Rossignol P, Le Coz S, Chedid A, et al. (2006) Blood pressure and renal outcomes in patients with kidney infarction and hypertension. J Hypertens 24(8): 1649-1654.
- Osler W (1901) The principles and practice of medicine: designed for the use of practitioners and students of medicine. (4th edtn), D. Appleton and Company, New York. 1182.
- Lawrence YR, Pokroy R, Berlowitz D, Aharoni D, Hain D, et al. (2010) Splenic infarction: an update on William Osler's observations. Isr Med Assoc J 12(6): 362-365.
- Antopolsky M, Hiller N, Salameh S, Goldshtein B, Stalnikowicz R (2009) Splenic infarction: 10 years of experience. Am J Emerg Med 27(3): 262-265.
- Ebert EC, Nagar M, Hagspiel KD (2010) Gastrointestinal and hepatic complications of sickle cell disease. Clin Gastroenterol Hepatol 8(6): 483-489.
- O'Keefe JH Jr, Holmes DR Jr, Schaff HV, Sheedy PF 2nd, Edwards WD (1986) Thromboembolic splenic infarction. Mayo Clin Proc 61(12): 967-972.
- Urban BA, Fishman EK (1998) Helical CT of the spleen. AJR Am J Roentgenol 170(4): 997-1003.
- Balcar I, Seltzer SE, Davis S, Geller S (1984) CT patterns of splenic infarction: a clinical and experimental study. Radiology 151(3): 723-729.
- Afzal A, Noor HA, Gill SA, Brawner C, Stein PD (1999) Leukocytosis inAcute Pulmonary Embolism. Chest 115(5): 1329-1332.
- Venetz C, Labarere J, Jimenez D, Aujesky D (2013) White blood cell count and mortality in patients with acute pulmonary embolism. Am J Hematol 88(8): 677-681.