Real Life Clinical Effectiveness of Razumab® (World’s First Biosimilar Ranibizumab) in Wet Age-Related Macular Degeneration: A Subgroup Analysis of Pooled Retrospective RE-ENACT Study
(Major) Sharma S1*, RE-ENACT Study Investigators Group2, Khan MA3, Chaturvedi A3
1 Medical Lead, Ophthalmology, Medical Services, Intas Pharmaceuticals Ltd. Ahmedabad, Gujarat, India.
2 RE-ENACT Study Investigators Group.
3 Medical Services, Intas Pharmaceuticals Ltd. Ahmedabad, Gujarat, India.
*Corresponding Author
Dr. (Major) Shashikant Sharma,
Medical Lead – Ophthalmology, Medical Services,
Intas Pharmaceuticals Ltd. Ahmedabad, Gujarat, 380054, India.
Tel: (O) +91-79-39837986
Mobile: +91 9998075884
E-mail: shashikant_sharma@intaspharma.com
Received: January 10, 2018; Accepted: February 10, 2018; Published: February 16, 2018
Citation: (Major) Sharma S, RE-ENACT Study Investigators Group, Khan MA, Chaturvedi A. Real Life Clinical Effectiveness of Razumab® (World’s First Biosimilar Ranibizumab) in Wet Age-Related Macular Degeneration: A Subgroup Analysis of Pooled Retrospective RE-ENACT Study Int J Oph thalmol Eye Res. 2018;6(2):368-373. doi: dx.doi.org/10.19070/2332-290X-1800074
Copyright: (Major) Sharma S© 2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: This subgroup analysis of RE-ENACT study (retrospective, multicenter, observational pooled study on wet age-related macular degeneration [wet AMD], diabetic macular edema, and retinal vein occlusion) evaluated the effectiveness of Razumab® (world’s first biosimilar ranibizumab by Intas Pharmaceuticals Ltd., India) in Indian patients with wet AMD.
Methods: Data of the patients with wet AMD, who were treated with ≥3 injections of Razumab® between January and August 2016, were included. Endpoints were: improvement in best corrected visual acuity (BCVA, measured by logMAR/ Snellen’s chart), decrease in central macular thickness (CMT, measured by Spectral Domain Optical Coherence Tomography), and intraretinal fluid (IRF) and subretinal fluid (SRF) from baseline at Weeks 4, 8 and 12.
Result: Medical charts of 194 patients were analysed; 112 (57.73%) were men, 82 (42.27%) were women. Mean ± SE BCVA improved from baseline (0.81 ±0.03) at Week 4 (0.78 ± 0.03; p=0.13) and attained significance at Weeks 8 (0.66 ± 0.02) and 12 (0.55 ± 00.02; p<0.0001 for both time points); mean ± SE CMT significantly decreased from baseline (393.02 ± 7.32 μm) to Weeks 4 (385.93 ± 7.10 μm; p=0.0041), 8 (332.75 ± 6.10 μm; p<0.0001) and 12 (293.5 ± 4.10 μm; p<0.0001). Proportion of patients with IRF and SRF significantly (P<0.0001) decreased from baseline to Weeks 4, 8 and 12 (59.79% vs. 47.94%, 41.75%, and 31.96%, respectively for IRF; and 82.47% vs. 66.49%, 51.03%, 41.24%, respectively for SRF). No new safety concerns with biosimilar ranibizumab were observed.
Conclusion: Razumab®(biosimilar ranibizumab) effectively improved the visual acuity and disease outcomes with no new safety concerns in patients with wet age-related macular degeneration in the real world setting.
2.Introduction
3.Materials and Methods
3.1 Study Population
3.2 Study Design
3.3 Study Assessments
3.4 Statistical Analysis
4.Results
4.1 Patients Disposition and Demographics
4.2 Endpoints
5.Discussion
6.Conclusion
7.Acknowledgments
8.Funding Information
9.Conflict of Interest
10.RE-ENACT Study Investigators Group
11.References
Keywords
Razumab; Efficacy; Safety; Wet Age Related Macular Degeneration; Wet AMD.
Introduction
Wet age-related macular degeneration (wet AMD), a leading cause of vision loss globally, is a late-onset, multifactorial retinal degenerative disease. The characteristic features of wet AMD are neovascularization originating from the choroidal vasculature and spreading to the subretinal pigment epithelium or subretinal space, and increased intraretinal (IRF) or subretinal fluid (SRF) [1-4]. The increased choroidal neovascularization (CNV), IRF and SRF are associated with over expression of vascular endothelial growth factor (VEGF)-A, which promotes neovascularization and leakage leading to wet AMD [5, 6].
Overall, AMD accounts for 8.7% vision loss cases globally and is the leading cause of irreversible vision loss in developed countries [7]. Wet type AMD corresponds to ~10-20% of all AMD cases, however, owing to the rapid progression and destructive nature, it is the major cause for irreversible blindness with a vision loss of ~80% [4, 8, 9]. AMD is an emerging public health problem in the developing world due to rapid increase in the ageing population with a prevalence of 3.1% to 10.6% [8]. In India, several population- based eye surveys have demonstrated that AMD accounts for 1.7-3.3% of all blindness diagnosed [10].
The treatment of wet AMD included laser photocoagulation and photodynamic therapy (PDT) from the 1980s to the early 2000s until the role of VEGF’s in the pathogenesis was well understood, and anti-VEGF became favorable [4]. The anti-VEGF agents facilitate the direct and selective inhibition of VEGF isoforms. Currently, ranibizumab and aflibercept are anti-VEGFs approved in the treatment of wet AMD. The most commonly used agent ranibizumab is a recombinant humanized monoclonal antibody, which binds to VEGF-A, and thus, prevents and hinders its interaction with vascular receptors VEGFR1 and VEGFR2 on the surfaces of vascular endothelial cells and reduces the endothelial cell proliferation, vascular leakage, and new blood vessel formation [5, 6, 11]. Two pivotal phase 3 studies MARINA [12] and ANCHOR [13] have demonstrated that ranibizumab therapy is associated with clinically and statistically significant benefits with respect to visual acuity, and is well-tolerated in the treatment of wet AMD.
Ranibizumab has been approved for the treatment of wet AMD by the US Food and Drug Administration in the year 2006, and European Medicine Agency in the year 2007. Razumab® (world’s first biosimilar ranibizumab from Intas Pharmaceuticals Ltd.) was developed to provide a cost-effective alternative to innovator ranibizumab, which has shown similar physicochemical characteristics, pharmacokinetics (PK), pharmacodynamics (PD) profile and clinical safety and efficacy, and has been approved by the Drug Controller General of India (February 2015) for the treatment of wet AMD, diabetic macular edema (DME), macular edema following retinal vein occlusion (RVO), and visual impairment due to CNV secondary to pathological myopia.
Prospective, randomized, double-masked, clinical trials have provided strong evidence of the efficacy and safety of ranibizumab in the treatment of wet AMD. A prospective study in Indian patients has also established the efficacy and tolerability of Razumab® in patients with chorioretinal vascular diseases including wet AMD [14]. Furthermore, a retrospective data collection study RE-ENACT was conducted to provide the ‘real-world’ clinical experience on the effectiveness of Razumab® in patients with wet AMD, DME and RVO. This report presents the subgroup analysis in patients with wet AMD on the effectiveness of Razumab® n real life clinical scenario.
This subgroup analysis included the patients of either sex, aged ≥18 years, with wet AMD, who received a minimum of three injections of biosimilar ranibizumab between January 2016 and August 2016 as part of their clinical care. Patient’s confidentiality was maintained throughout at all points of the data analysis.
The study protocol was approved by the Independent Ethics Committee ‘Inter system Biomedica Ethics Committee’, Mumbai. The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki, and in accordance with the International Conference on Harmonization’s Good Clinical Practice guidelines, applicable regulatory requirements, and in compliance with the protocol.
This was a subgroup analysis on wet AMD patients from RE-ENACT study, which was a retrospective, multicenter, organized, observational study that analyzed the medical charts of patients who were administered a minimum of 3 intravitreal biosimilar ranibizumab injections (4-weekly) between January 2016 and August 2016 at 16 centers in India. Patients, both treatment naïve or treated with other anti-VEGF/steroids/laser treatment, were included in the study. Patients with dense cataract were excluded where optical coherence tomography (OCT) assessment was not possible.
The primary endpoints were the mean change in the best corrected visual acuity (BCVA), measured by Snellen’s chart or LogMar chart; mean change in the central macular thickness (CMT), measured by spectral domain OCT (SD-OCT); and proportion of patients showing no IRF and SRF measured by SD-OCT from baseline to Weeks 4, 8 and 12 endpoints.
Demographic and baseline characteristics were summarized using descriptive statistics. Categorical variables were summarized with frequency and percentage. Continuous variables were summarized with count, mean, standard deviation, median, minimum and maximum. Best corrected visual acuity and central macular thickness data were analyzed using two-tailed paired t-test. Intraretinal fluid and sub-retinal fluid data was analyzed using χ2 test. Mean % change in BCVA and CMT was calculated as an average value of % change from baseline to a particular visit. All statistical analyses were done using SAS 9.3 or higher.
The subgroup analysis included 194 patients (112 men and 82 women) with wet AMD, who received a minimum of 3 intravitreal biosimilar ranibizumab injections (4-weekly) treatment between January 2016 and August 2016. Disease history included arthritis (n=2), tuberculosis (n=2), and cholesterol, joint pain, Koch’s disease, and ischemic heart disease (each in 1 patient). Majority (14.95%) of the patients had Classic, Subfoveal type wet AMD followed by Classic, Extrafoveal (11.86%); 54.6% (106/194) patients had diabetes and 55.6% (108/194) had hypertension. The baseline characteristics of the patients are summarized in Table 1.
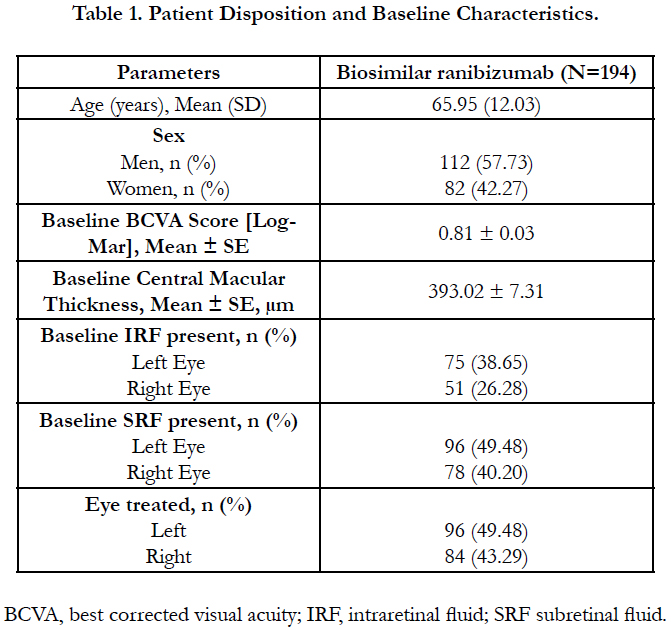
The mean ± SE BCVA improved from baseline (0.81 ± 0.03) at Week 4 (0.78 ± 0.03; p=0.13) and attained significance at Weeks 8 (0.66 ± 0.02) and 12 (0.55 ± 0.02; p<0.0001 for both time points). Biosimilar ranibizumab led to a rapid and continuous improvement in visual acuity measured by logMAR BCVA, with benefits observed as early as Week 4 (Figure 1).
Figure 1. Mean ± SE BCVA (logMAR) at baseline and Weeks 4, 8 and 12 after biosimilar ranibizumab administration.
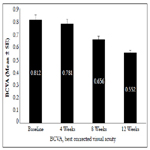
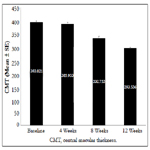
A significant decrease in the mean ± SE CMT scores indicating improved disease condition was observed from baseline (393.02 ± 7.31 μm) to Weeks 4 (385.93 ± 7.10 μm; p=0.0041), 8 (332.75 ± 6.10 μm; p<0.0001) and 12 (293.5 ± 4.10 μm; p<0.0001) (Figure 2).
Figure 2. Mean CMT (μm) at baseline and Weeks 4, 8 and 12 after biosimilar ranibizumab administration.
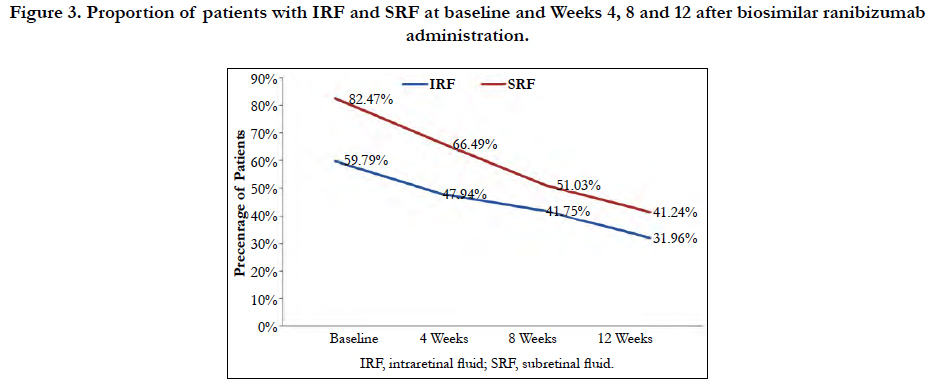
A significant reduction in the proportion of patients having IRF or SRF from baseline to all the time points (Weeks 4, 8 and 12) were observed, indicating improved disease condition. The percentage of patients having IRF at baseline (59.79%) reduced at Weeks 4, 8 and 12 to 47.94%, 41.75%, and 31.96%, respectively (p<0.001 for all). Similarly, the percentage of patients having SRF at baseline (82.47%), reduced at Weeks 4, 8 and 12 to 66.49%, 51.03%, 41.24%, respectively (p<0.001 for all) (Figure 3).
Figure 3. Proportion of patients with IRF and SRF at baseline and Weeks 4, 8 and 12 after biosimilar ranibizumab administration.
Discussion
This retrospective subgroup analysis from RE-ENACT study, pooled data of patients with wet AMD, DME and RVO sought to investigate the ‘real-world’ clinical use of biosimilar ranibizumabin the treatment of patients with wet AMD. The results showed that BCVA and CMT were significantly improved from baseline indicating improvement in the visual acuity and disease condition after the 1st injection of biosimilar ranibizumab (Week 4), which was maintained at the 3rd injection, at Week 12. Findings of the present study on biosimilar ranibizumab are consistent with the original pooled data as well as several reported studies that demonstrate the effectiveness of ranibizumab in patients with wet AMD.
Several multicenter, randomized, prospective studies have demonstrated that the anti-VEGF agents are an effective treatment option for wet AMD, DME, RVO, and myopic choroidal neovascularization, and ranibizumab is considered the gold standard treatment for the majority of these pathological entities [15]. In the 24-month MARINA and ANCHOR studies, ranibizumab showed sustained benefits in the treatment of wet AMD. For the long-term prognosis of wet AMD, ranibizumab demonstrated a revolutionary vision gain in the randomized, placebo-controlled, double-blind MARINA trial (n=716). Visual acuity was improved with ranibizumab treatment at 12-months, and the benefits were maintained at 24 months [16]. The randomized, double-blind ANCHOR trial (n=423) established the long-term superiority of ranibizumab over verteporfin at 12-months follow-up [13].
In our study, a significant improvement in the logMar BCVA scores at 12 weeks after biosimilar ranibizumab therapy was observed, which is consistent with a single-center, retrospective observational study with a 6-month follow-up in Chinese patients with mean BCVA (number of ETDRS letters) increase from 43.2 ± 19.3 at baseline to 51.7 ± 20.1 at 12 months (p<0.001) [5]. Similarly, Figurska and Stankiewicz showed a significant improvement in the visual acuity (logMar BCVA) from baseline (0.73 ± 0.27 logMAR) to after the third ranibizumab injection (0.54 ± 0.27 logMAR) [17]. A retrospective study in 79 patients with exudative AMD showed a significant increase in the mean BCVA scores from baseline (0.78 ± 0.33) to 12-months (0.61 ± 0.39, p<0.001) [18].
Improvements in the visual acuity and functional and anatomical visual improvement is demonstrated with the initial ranibizumab administration itself [19], which was also reported in our analysis with a marked improvement in the BCVA, CMT, and a decrease in the percentage of patients with SRF and IRF as early as 4 weeks following the first biosimilar ranibizumab dose. Further, there was a significant improvement (p<0.0001) in these parameters till Week 12. Yun and colleagues demonstrated a significant initial response of ranibizumab for improvement in logMAR BCVA in 39 patients with wet AMD from baseline (0.73 ± 0.45) to 3-months (0.50 ± 0.38), results for improvement in BCVA profile is similar to the current study confirming the efficacy of initial biosimilar ranibizumab administration [20]. Furthermore, the UK real-life study for ranibizumab in wet AMD showed improvement in mean BCVA (ETDRS) after 3 ranibizumab injections (at 3-months) [21]. The thickness of central macula corresponds to the ganglion cell damage. The current study demonstrated a significant decrease in the CMT with biosimilar ranibizumab treatment indicating the improved disease outcomes. Ranibizumab treatment has shown a significant decrease in CMT from baseline at 3 months (320 μm vs. 265 μm) after treatment in a retrospective study (n=50) by Castiblanco and Adelman [22]. Another study by Biswas and colleagues has shown results similar to our study for average decrease in CMT indicating improvement in disease condition from baseline to 3 months (288.63 vs. 217.07 μm; p<0.001) in patients receiving ranibizumab [23]. Similar results for decrease in CMT are reported in earlier published studies in real-life setting as well as retrospective chart review [24-26]. The current study demonstrates the effects of biosimilar ranibizumab on the functional and anatomical visual improvement as marked improvements seen in the BCVA and CMT at Week 4 after the first injection, and significant improvement at Weeks 8 and 12. The degree of improvements in BCVA and CMT at Weeks 8 and 12 after intravitreal biosimilar ranibizumab injections were similar to those reported for pooled data as well as in reported studies with ranibizumab.
Due to the abnormal choroidal neovascularization causing the leakage of fluid into and underneath the neurosensory retina, wet AMD typically presents with IRF and SRF. Ranibizumab has shown to decrease IRF and SRF in wet AMD patients. A Prospective cohort study within a randomized clinical trial demonstrated that ranibizumab treatment was effective in reducing the proportion of patients with IRF and SRF starting at 4 weeks, which was maintained till 2 years follow-up [27]. The RE-ENACT study, original retrospective pooled study, did not capture the complete information on adverse events in the medical records and hence, not analyzed in this subgroup. Overall, no new safety concerns compared to innovator product were observed.
Conclusion
From the pooled RE-ENACT, a retrospective, observational study on wet age-related macular degeneration, diabetic macular edema, and retinal vein occlusion, the current subgroup analysis in patients with wet age-related macular degeneration showed that intravitreal injection of Razumab®, world’s first biosimilar ranibizumab from Intas Pharmaceuticals Ltd, is effective in reducing macular thickness and improving visual acuity in a real-world setting.
Acknowledgments
The authors thank Mr. Shreekant Sharma (Lambda Therapeutic Research Ltd.) for providing writing assistance and Dr. Venugopal Madhusudhana (Lambda Therapeutic Research Ltd.) for additional editorial assistance for the development of this manuscript. The authors also thank the clinical data management and biostatistics department of Lambda Therapeutic Research Ltd. For their contribution in the preparation of statistical analysis report in this retrospective data collation and analysis. The authors are thankful to Dr. Ramandeep Sharma (Intas Pharmaceuticals Ltd.) for conceptualization of the RE-ENACT study. The authors also thank Mr Tanishq Sharma (Shree Krishna Hospital and Pramukhswami Medical College, Karamsad, Anand, Gujarat, India) for conceptualization of the RE-ENACT study and also providing support for data collection and analysis.
Funding Information
The authors received honorarium from Intas Pharmaceuticals Ltd., for data collection.
Conflict of Interest
Drs. Shashikant Sharma, Mujtaba Khan and Alok Chaturvedi are employees of Intas Pharmaceuticals Ltd, Ahmedabad.
RE-ENACT Study Investigators Group
Dr .Manjunath Bhaskar Anandkumar, MS; Ganesh Netralaya-Sirsi, Karnataka, India, 581401. Email: manj009@gmail.com
2. Dr. Naresh Yadav, FRCS; NarayanaNethralaya Bengaluru, Karnataka, India, 560010. Email: vasudha.naresh@gmail.com
3. Dr. Vaibhav Shrivastava, MS; Shradha Eye Care, Kolkata, West Bengal, India, 700038. Email: vaibhav.shrivastava.28@gmail.com
4. Dr. PasupathyElankumaran, MD; NavasakthiNetralaya Bengaluru, Karnataka, India, 560043. Email: drelankumaran@gmail.com
5. Dr. Shrinivas Joshi, MS; M M Joshi Eye Institute Hubli, Karnataka, India, 580031. Email: shrinivasmjoshi@gmail.com
6. Dr. Subijay, MD; Susrut Eye Hospital Kolkata, West Bengal, India, 700106. Email: subijay.sinha@gmail.com
7. Dr. Ananyabrata Das, DOMS; Spectra Eye Hospital Kolkata, West Bengal, India, 700136. Email: drananyabrata@yahoo.com
8. Dr. Naveenam Srinivasa Muralidhar, MS; Retina Institute of Karnataka, Bengaluru, Karnataka, India, 560018. Email: retina.nsm@gmail.com
9. Dr. Sangita Jain, MS; Dev Bhumi Superspeciality Hospital, Dehradun, Uttarakhand, India, 248001. Email: sangitavrs@yahoo.co.uk
10. Dr. Prosenjit Mondal, MS; Susrut Eye Hospital Kolkata, West Bengal, India, 700106. Email: drprosenjit2007@gmail.com
11. Dr.Hemanth Murthy, MS; Retina Institute of Karnataka Bengaluru, Karnataka, India, 560018. Email: hemanthmurthy@yahoo.com
12. Dr. Alay Banker, MS; Bankers Retina Clinic Ahmedabad, Gujarat, India, 380014. Email: alay.banker@gmail.com
13. Dr. Nishikant Borse, MS; Insight Eye Clinic Mumbai, Maharashtra, India, 400014. Email: nishikantborse@yahoo.com
14. Dr. GirishRao, MS; Sri Ganpat iNetralaya Jalna; Maharashtra, India, 431203. Email: drgr1968@gmail.com
15. Dr. Rajender Pal Singh, MD; Visitech Eye Centre Delhi, India, 110025. Email: rp@visitech.org
16. Dr. GunjanPrakash, MD; SPG Medicare & Diagnostics Agra, Uttar Pradesh, India, 282005. Email: gunjanprakash@gmail.com
References
- Friedman DS, O'Colmain BJ, Muñoz B, et al; Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004 Apr;122(4):564-72. PubMed PMID:15078675.
- Wong WL, Su X, Li X, Cheung CM, Klein R, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014 Feb;2(2):e106-16. doi: 10.1016/S2214-109X(13)70145-1. Epub 2014 Jan 3. PubMed PMID:25104651.
- Jaffe GJ, Martin DF, Toth CA, Daniel E, Maguire MG, et al. Macular Morphology and Visual Acuity in the Comparison of Age-related Macular Degeneration Treatments Trials (CATT). Ophthalmology.2013S ep;120(9):1860-70.doi:10.1016/j.ophtha.2013.01.073. Epub 2013 May 1. PubMed PMID: 23642377.
- Topal T, Kar T, Yıldırım Y, Sağdıç SK, Büyükavşar C, et al. Evaluation of Aflibercept Treatment Responses in Eyes with Bevacizumab/Ranibizumabresistant Wet Age-related Macular Degeneration. Turk J Ophthalmol. 2017 Jun;47(3):133-137. doi: 10.4274/tjo.34735. Epub 2017 Jun 1. PubMed PMID: 28630787.
- Qi HJ, Li XX, Zhang JY, Zhao MW. Efficacy and safety of ranibizumab for wet age-related macular degeneration in Chinese patients. Int J Ophthalmol. 2017 Jan 18;10(1):91-97. doi: 10.18240/ijo.2017.01.15. eCollection 2017. Pubmed PMID: 28149783.
- Dervenis N, Mikropoulou AM, Tranos P, Dervenis P. Ranibizumab in the Treatment of Diabetic Macular Edema: A Review of the Current Status, Unmet Needs, and Emerging Challenges. Adv Ther. 2017 Jun;34(6):1270-1282. doi: 10.1007/s12325-017-0548-1. Epub 2017 May 8. PubMed PMID: 28484955.
- Resnikoff S, Pascolini D, Mariotti SP, Pokharel GP. Global magnitude of visual impairment caused by uncorrected refractive errors in 2004. Bull World Health Organ. 2008 Jan;86(1):63-70. PubMed PMID: 18235892.
- Thapa R, Bajimaya S, Paudyal G, Khanal S, Tan S, et al. Prevalence of and risk factors for age-related macular degeneration in Nepal: the Bhaktapur Retina Study. Clin Ophthalmol. 2017 May 22;11:963-972. doi: 10.2147/OPTH.S132338. eCollection 2017. PubMed PMID: 28579747.
- Bressler NM. Early detection and treatment of neovascular age-related macular degeneration. J Am Board Fam Pract. 2002 Mar-Apr;15(2):142-52. PubMed PMID: 12002198.
- Woo JH, Sanjay S, Au Eong KG. The epidemiology of age-related macular degeneration in the Indian subcontinent. Acta Ophthalmol. 2009 May;87(3):262-9. doi: 10.1111/j.1755-3768.2008.01376.x. Epub 2008 Nov 12. PubMed PMID: 19016663.b.
- Lucentis Prescribing Information. Genentech Inc, CA. April 2017.
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, et al. Ranibizumab for Neovascular Age-Related Macular Degeneration. N Engl J Med. 2006 Oct 5;355(14):1419-31. PubMed PMID: 17021318.
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006 Oct 5;355(14):1432-44. DOI: 10.1056/NEJMoa062655. PubMed PMID: 17021319.
- Sameera VV, Apoorva AG, Joshi S, Guruprasad AS. Safety and efficacy of Razumab-The new biosimilar in India: Our experience. Kerala Journal of Ophthalmology. 2016 Sep 1;28(3):180.
- Triantafylla M, Massa HF, Dardabounis D, Gatzioufas Z, Kozobolis V, et al. Ranibizumab for the treatment of degenerative ocular conditions. Clin Ophthalmol. 2014 Jun 24;8:1187-98. doi: 10.2147/OPTH.S40350. eCollection 2014. PubMed PMID: 25028531.
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006 Oct 5;355(14):1419-31. DOI: 10.1056/NEJMoa054481. PubMed PMID: 17021318.
- Figurska M, Stankiewicz A. Effectiveness of ranibizumab intravitreal injections for exudative age-related macular degeneration treatment: 12-month outcomes. Med Sci Monit: International Medical Journal of Experimental and Clinical Research. 2011 Sep;17(9):CR485-90. doi: 10.12659/MSM.881934. PubMed PMID: 21873944.
- Querques G, Azrya S, Martinelli D, Berboucha E, Feldman A, et al. Ranibizumab for exudative age-related macular degeneration: 24-month outcomes from a single-centre institutional setting. Br J Ophthalmol. 2010 Mar;94(3):292-6. doi: 10.1136/bjo.2009.170670. Epub 2009 Dec 1. PubMed PMID: 19951942.
- Bolz M, Simader C, Ritter M, Ahlers C, Benesch T, et al. Morphological and functional analysis of the loading regimen with intravitreal ranibizumab in neovascular age-related macular degeneration. Br J Ophthalmol. 2010 Feb;94(2):185-9. doi: 10.1136/bjo.2008.143974. Epub 2009 Aug 18. PubMed PMID: 19692384.
- Yun C, Oh J, Choi KE, Hwang SY, Kim SW, et al. Peripapillary choroidal thickness after intravitreal ranibizumab injections in eyes with neovascular age-related macular degeneration. BMC Ophthalmology. 2016 D;16(1):25. DOI: 10.1186/s12886-016-0203-7.
- Vardarinos A, Gupta N, Janjua R, Iron A, Empeslidis T, et al. 24-month clinical outcomes of a treat-and-extend regimen with ranibizumab for wet age-related macular degeneration in a real life setting. BMC Ophthalmol. 2017 Apr 27;17(1):58. doi: 10.1186/s12886-017-0451-1. PubMed PMID: 28449645.
- Castiblanco CP, Adelman RA. Visual Acuity and Central Macular Thickness in Patients Treated With Anti-Angiogenic Intravitreal Injections for Age-Related Macular Degeneration. Investigative Ophthalmology & Visual Science 2018 May 14;49(13):267.
- Biswas P, Sengupta S, Choudhary R, Home S, Paul A, et al. Comparative role of intravitreal ranibizumab versus bevacizumab in choroidal neovascular membrane in age-related macular degeneration. Indian J Ophthalmol. 2011 May-Jun;59(3):191-6. doi: 10.4103/0301-4738.81023. PubMed PMID: 21586838.
- Boulanger-Scemama E, Sayag D, Ha Chau Tran T, Quaranta-El Maftouhi M, Rumen F, et al. [Ranibizumab and exudative age-related macular degeneration: 5-year multicentric functional and anatomical results in reallife practice]. J Fr Ophtalmol. 2016 Oct;39(8):668-674. doi: 10.1016/j. jfo.2016.06.001. Epub 2016 Sep 5. PubMed PMID: 27609025.
- Zhu M, Chew JK, Broadhead GK, Luo K, Joachim N, et al. Intravitreal Ranibizumab for neovascular Age-related macular degeneration in clinical practice: five-year treatment outcomes. Graefes Arch Clin Exp Ophthalmol. 2015 Aug;253(8):1217-25. doi: 10.1007/s00417-014-2799-8. Epub 2014 Sep 10. PubMed PMID: 25205618.
- Suthar T, Lieberman RM, Rhee DY. Effect of Intravitreal Ranibizumab (Lucentis®) on the Foveal and Macular Thickness in the Contralateral Eye in Patients with Neovascular Age Related Macular Degeneration. Investigative Ophthalmology & Visual Science. 2012 Mar 26;53(14):5160-5160.
- Sharma S, Toth CA, Daniel E, Grunwald JE, Maguire MG, et al. Macular Morphology and Visual Acuity in the Second Year of the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2016 Apr;123(4):865-75. doi: 10.1016/j.ophtha.2015.12.002. Epub 2016 Jan 9. PubMed PMID: 26783095.