Long-Term Efficacy of Photorefractive Keratectomy In Hyperopia Patients
Ulusoy DM*
Kayseri Training and Research Hospital, Department of Ophthalmology, Kayseri, Turkey.
*Corresponding Author
Döndü Melek Ulusoy, MD,
Kayseri Training and Research Hospital,
Department of Ophthalmology, Kayseri, Turkey.
Tel: +905303273827
E-mail: melek_ern@hotmail.com
Received: January 08, 2018; Accepted: February 10, 2018; Published: February 16, 2018
Citation: Ulusoy DM. Long-Term Efficacy of Photorefractive Keratectomy In Hyperopia Patients. Int J Ophthalmol Eye Res. 2018;6(2):363-367. doi: dx.doi.org/10.19070/2332-290X-1800073
Copyright: Ulusoy DM© 2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: To review the long-term efficacy, stability and reliability of photorefractive keratectomy (PRK) treatment in hyperopia patients and to assess the complications that may arise during the follow-up period.
Materials and Methods: 76 eyes of 42 patients who underwent PRK treatment for hyperopia were included in this retrospective study. Patients were separated into two groups according to their spherical equivalent (SE) values. Both groups were analyzed and compared in terms of post-operative refraction and vision acuity, post-operative complications, keratometry values, stabilization period and the results of interventions for treatment.
Results: Mean age of the patients was 33.97 ± 10.26 (18-51) years and the mean follow-up period was 27.16 ± 8.43 (6-39) months. Preoperative mean SE value was 1.64 ± 0.76D in group 1 and +4.45 ± 1.18D in group 2. In the last follow-up examination, those values were measured as +0.20 ± 1.01D and +1.56 ± 1.37D respectively. Uncorrected visual acuity (UCVA) of both groups were significantly higher in the last control in comparison with pre-PRK period (p=0.002 in group 1, p=0.001 in group 2). After 6 months, 92.5% of the eyes in group 1 and 85.6% of the eyes in group 2 were within ± 1.00 D range. Forty-seven (61.8%) eyes had no corneal haze meanwhile 15 (19.7%) had grade 1, 11(14.4%) had grade 2 and 3(3.9%) had grade 3 corneal haze. There was no difference in terms of haze formation between the groups (p=0.183).
Conclusion: In long term, PRK treatment is an efficient, reliable and predictable method that can be used in correcting hyperopia.
2.Introduction
3.Methods
3.1 Surgical Method
3.2 Statistical Analysis
4.Results
4.1 Refraction
4.2 Visual Acuity
4.3 Central Cornea Thickness (CCT)
4.4 Haze Formation
4.5 Stabilization Period
4.6 Keratometry
4.7 Efficacy
4.8 Reliability
4.9 Predictability
5.Discussion
6.Conclusion
7.References
Keywords
Excimer Laser; Photorefractive Keratectomy; Hypermetropia.
Introduction
Hyperopia is a common refractive error that is usually corrected with eyeglasses or contact lenses. Following the fast advancement in refractive surgery and technology, eyeglasses or contact lenses were no longer the single treatment option in refractive errors. Today, extraction of transparent lens and intraocular lens implantation, phacic intraocular lens applications and corneal surgeries are performed for correction and treatment of refractive errors. Excimer Laser is the most widely used method in refractive surgery methods. In this method, cornea is reshaped using 193 nm argon-flourine laser and cornea is corrected according to patient’s refractive error. Different techniques can be chosen for patients with differences in cornea structure, refraction errors and patient expectations. The most frequently used methods are PRK (Photorefractive keratectomy), LASIK (Laser in situ keratomileusis) and LASEK (Laser subepithelial keratomileusis) [1, 2].
PRK is the first widely used excimer laser method. In this method, cornea epithelium is mechanically peeled and then excimer laser is applied. PRK is used as a safe, efficient and predictable method in correction of myopia and astigmatism as well as treatment of hyperopia. However, post-operative pain, corneal haze formation, high regression rate and late stabilization of visual acuity in patients with high grade hyperopia (>+6.00) are the disadvantages of this method [3, 4].
The main objective of this study was to investigate the long-term efficacy, predictability, stability and reliability of PRK applications in hyperopia patients by analyzing pre-op and post-op refraction values, visual acuity and possible post-operative complications.
Methods
Seventy-six eyes of 42 patients that received PRK treatment in our clinic’s Corneal and Refractive Surgery department were retrospectively reviewed. Patients without regular control appointments and with follow-up periods less than 6 months were not included in the study.
All patients underwent a complete ophthalmological examination before operation including uncorrected visual acuity (UCVA) and best corrected visual acuity (BCVA) measurement (converted into Logarithm of the Minimum Angle of Resolution [logMAR] to be used in statistical analysis), refraction measurement (manifest and with cycloplegia), and fundus examination with dilation using bio-microscope were performed. Keratometry values (K), corneal topography, central corneal pachymetry and pupil diameter were measured using Sirius 3D Rotating Scheimpflug Camera-Topography System (Costruzione Strumenti Oftalmici, Florence, Italy). Pneumatic tonometer was used to measure intraocular pressure. Patients included in the study were divided into 2 groups according to their spherical equivalent (SE) values. Group 1 included 14 eyes with low grade hyperopia and/or hyperopia with astigmatism (SE value +1.00 diopters [D] to + 2.99 diopters) and group 2 had 42 eyes with high grade hyperopia and/or hyperopia with astigmatism (SE value +3.00 to +6.50 D). Both groups were analyzed in terms of post-operative refraction, UCVA, BCVA, post-operative corneal haze grades, keratometry values (Sim K1= Simulated keratometry in the steepest meridian, Sim K2 = Simulated keratometry in the flattest meridian), stabilization period and results of treatment interventions. All patients were informed about the risks and present alternatives for surgical procedure and a written consent was taken.
Corneal haze evalution was graded using the following objective criteria using the slit-lamp biomicroscopia:
Grade 0: Completely clear cornea
Grade 1: Traces of reticular subepithelial haze visible only with broad tangential illumination
Grade 2: Clearly visible reticular subepithelial haze diffusely distributed
Grade 3: Grade 2 haze with areas of confluence
Grade 4: Dence opacity completely obscuring details of intraocular structures.
All patients were operated by the same surgeon (NC). First, after administering topical proparacaine 0.5%; (Alcaine, Alcon) and disinfection of the skin surface area around the eyes with povidone-iodine solution, area was draped and lid speculum was positioned. 8.0-9.00 corneal trephine was positioned over cornea. The container was filled with 20% ethyl alcohol and kept for 20 seconds. After that, the alcohol was absorbed using cellulose sponge and cornea was washed with BSS. Epithelium was lifted and separated from incision edges using crescent blade. After drying stroma, laser ablation was performed (SCHWIND ESIRIS excimer laser's ORK-CAM software [SCHWIND eye-tech solutions, Kleinostheim, Germany]). An antibiotic eye drop ofloxacin 0.3%; (Exocin, Allergan) was administered and a therapeutic contact lens was fitted. Topical antibiotic eye drops ofloxacin 0.3%; (Exocin, Allergan) qid and artificial tears hyaluronic acid 0.15%; (Eyestil, Teka) qid was used in post-operative period. Following the closure of corneal epithelium, contact lens was removed and topical fluoromethalone 0.1%; (Flarex, Alcon) qid was added to treatment regime. The treatment period was completed to one month and patients were followed in an outpatient basis on first week, first month, third month and sixth month of the operation and then in 6-month periods.
SPSS for Windows Version 15.0 programme was used for statistical analysis. Numerical variables were summarized with average ± standart deviation, median and minimum-maximum values; cathegorical variables were summarized with number and percentage. Continuous variables were compared with student's t-test and qualitative variables were compared with chi square test between groups. The level of significance was set at 0.05 with 95% confidence interval.
Results
Twenty-eight (69.73%) male and 14 (30.26%) female patients were included in the study. Mean age of the patients was 33.97 ± 10.26 years (18-51) and mean patient follow-up period was 27.16 ± 8.43 (6-39) months.
Results of patients were assessed separately for each group.
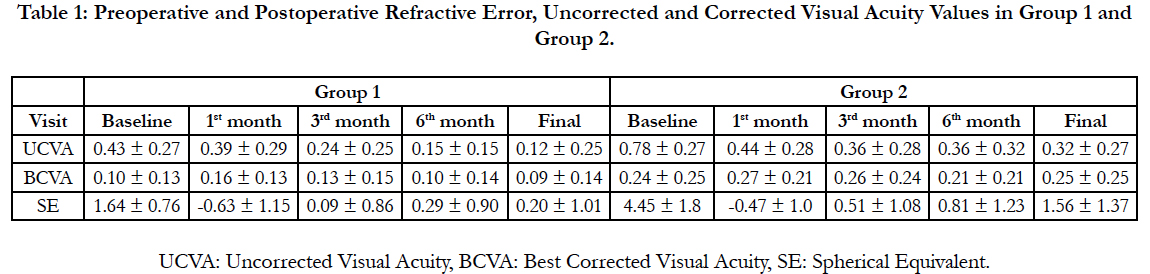
Table 1 shows the refraction status of the patients with time in SE values. Preoperative mean SE value in group 1 was +1.64 ± 0.76 D and postoperative mean SE values were -0.63 ± 1.15 D, +0.09 ± 0.86 D, +0.29 ± 0.90 D, +0.20 ± 1.01 D during first, third, sixth month of the operation and in final follow-up examination (Table 1). In group 2, those values were +4.45 ± 1.18 D in preoperative period and -0.47 ± 1.20 D, +0.51 ± 1.08 D, +0.81 ± 1.23 D and +1.56 ± 1.37 D during first, third, sixth month of the operation and in final follow-up examination (Table 1).
Table 1: Preoperative and Postoperative Refractive Error, Uncorrected and Corrected Visual Acuity Values in Group 1 and Group 2.
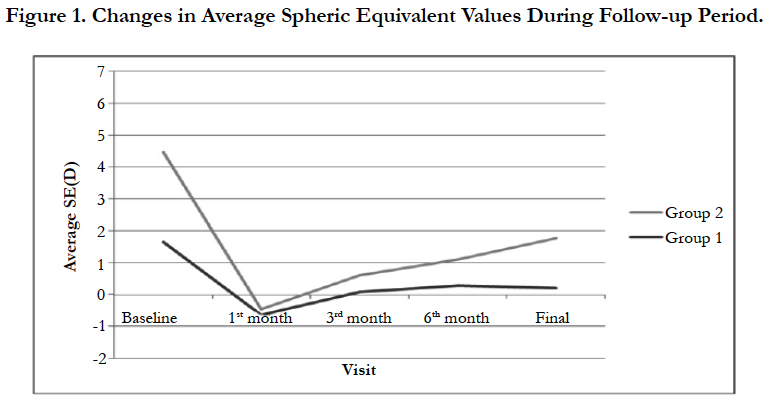
As seen in Table 1, during the first month of the operation both groups had myopic SE meanwhile in the third month; SE was closer to emetropia. After 3 months, a slight regression which is more significant in group 2 was detected (Figure 1).
Mean UCVA in both groups were significantly higher in the last control in comparison with pre-PRK period (p=0.002 in group 1, p=0.001 in group 2). There was no significant change in mean BCVA from preoperative to postoperative period (p=0.48 for group 1 and p=0.46 for group 2).
Eleven (78.57%) eyes in group 1 had no change in BCVA after the treatment meanwhile two (14.28%) had one line and one (7.14%) had a two line increase. In group 2, 48 (78.57%) eyes had no change whereas 6 (9.67%) eyes had one line and one (1.61%) eye had a two line increase. Four (6.45%) eyes had one line and 2 (3.22%) eyes had two line vision loss.
Mean preoperative CCT was 536.87 ± 36.86 μm in group 1 and 51.13 ± 25.85μm in group 2. During the last post-operative follow-up appointment, those values were measured as 533.93 ± 28.75 μm and 497.20 ± 36.42 μm in group 1 and group 2 respectively. This thinning in postoperative period was not statistically significant in both groups (p=0.15).
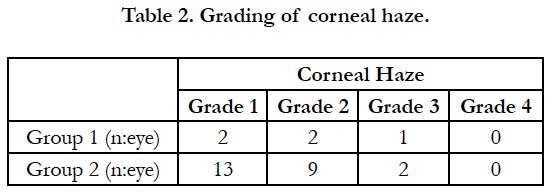
Forty-seven (61.84%) operated eyes had no haze while two (2.63%) eyes had grade 1, two (2.63%) eyes had grade 2, and one (1.31%) eye had grade 3 haze formation in group 1. In group 2, 13(17.10%) eyes had grade 1, 9(11.84%) eyes had grade 2 and two (2.63%)eyes had grade 3 corneal haze. There was no statistically significant difference in haze formation between the groups (p=0.183) (Table 2). Haze density was maximized on 1st to 3rd month of the operation and decreased on the 12th month.
Target refractive value was reached in 6.85 ± 7.7 months in group 1 and 4.67 ± 1.96 months in group 2. No significant difference was found between the groups in terms of target refractive value acquisition period (p=0.12).
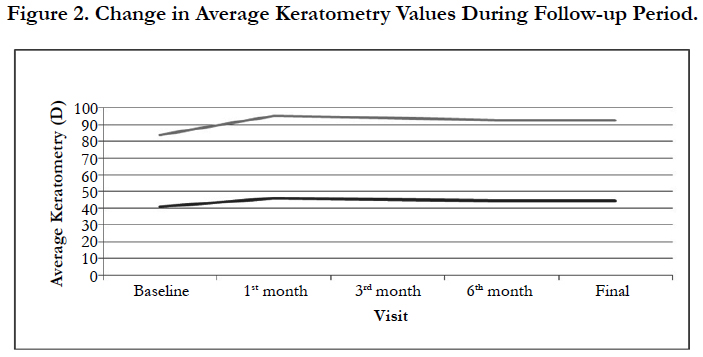
Mean keratometry values were 41.06 ± 1.12 D in group 1 and 42.92 ± 1.96 D in group 2. The highest measurements were obtained on the first month of the operation with 46.16 ± 2.88 D in group 1 and 49.07 ± 3.74 D in group 2. On the 3rd month, mean keratometry values were 45.28 ± 1.35 D and 48.62 ± 3.54 D in group 1 and group 2 respectively. Keratometry values showed stabilization after 6 months (6th month, group 1:44.58 ± 1.12 D, group 2: 47.84 ± 2.46 D) and it continued throughout to the last control appointments (Final: group 1:44.56 ± 1.16 D, group 2: 47.78 ± 2.28 D), (Figure 2).
Efficacy index was calculated as mean postoperative UCVA/mean preoperative BCVA and the results were 0.92 in group 1 and 0.79 in group 2.
Reliability index was calculated as mean postoperative CDVA/ mean preoperative CDVA and the results were 1.02 in group 1 and 0.98 in group 2.
Predictability was defined as the rate of cases which were targeted between ± 0.50 D and ±1.00 D. At the end of 6th month, Group 1 had 13 (92.5%) and group 2 had 53 (85.6%) eyes that were within ± 1.00 D range. In addition, 11 (82.4%) eyes in group 1 and 39 (62.8%) eyes in group 2 were within ± 0.50 D range (Figure 1).
Discussion
Surgical treatment of hypermetropia tails away from treatment of myopia because of the fact that steepening cornea is more difficult than flattening cornea. Several methods such as hexagonal keratectomy, radial and diode thermokeratoplasty, contact and non-contact Ho:YAG, conductive keratoplasty, automatic lamellar keratoplasty, clear lense extraction, intrastromal corneal implants were tried in treatment of hypermetropia. But low predictability, refractive stability and high complication rate were reported from these methods [4-6]. 193_nm excimer laser is used successfully in treatment of myopia and astigmatism [7, 8]. This laser, generates a smooth ablation on peripheral corneal stroma, could be used in hypermetropia treatment with its promising results [9].
The efficacy and reliability of PRK therapy in hypermetropic patients up to +5.00 D were documented in several studies [10-17]. It has been reported that in high hyperopia (˃5.00 D), PRK is a method that has less predictable results associated with higher regressions [18].
In our study, low and high grade hyperopia patients that underwent PRK were analyzed and compared in terms of post-operative refraction and visual acuity, post-operative complications, keratometry values, stabilization period and the results of interventions for treatment. Our study also complements the literature by giving long-term results of high patient rates. To sum up, PRK treatment is an efficient, reliable and predictable method even in long term that can be used in the treatment of hyperopia patients. A study by O’Brart et al., conducted on 40 hyperopic eyes that underwent PRK treatment, divided the subjects into 4 groups according to their preoperative refractive errors (group 1:+1.50 D, group 2:+3.00 D, group 3:+4.50 D, group 4:+6.00 D). Overcorrection was detected during the first month of the surgery. Emetropia was achieved between 3 to 6 months and refractive stability was reached at 12th month. Mean SE value at the 12th month was +0.55 D, which was measured as +0.83 D after 7.5 years of follow-up. Regression was not reported in any of the patients [11]. El-Agha et al., did a study on 22 PRK received hyperopic eyes (≤+6.00 D) and found overcorrection at the first month and a fast refractive regression and myopic neutralization 6 months after surgery. Refractive stability was achieved between 3 to 6 months [23]. Similar results were also reported on Jackson et al’s study [15]. Corones et al’s., study performed on 38 eyes with SE values between +1.00 D and +8.00 D revealed that starting with overcorrection during post-operative period, mean SE value was found to be ± 0.20 D at the end of follow-up period. Refractive stability was achieved in a period longer than a year [17]. In our study, both groups were diagnosed with low myopic refractive values on 1st month of the operation and values closer to emetropia were reached on 3rd month. After 3rd month, a slight regression especially prominent in group 2 was seen. Refractive stability was achieved in 6.85 months in group 1 and in 4.67 months in group 2. These results are deemed to be more successful with lower regression rates and shorter stabilization periods found in the literature.
Regression after hyperopic excimer laser surgery is thought to be caused by epithelial hyperplasia and stromal regrowth in ablation zone [19-21]. Hosoda et al., detected subepithelial proliferative changes in ablation zone during 1st month that was decreased during 3rd month of the operation in rabbit models examined with confocal microscopy and histological methods [20]. Reinstein et al., detected epithelial thickening in human eye following hyperopic LASIK surgery using high-frequency ultrasonography [19]. This effect was related with the dosage received and this was proved with high regression rates in high grade hyperopic refractive errors [13, 18, 19]. Clinically, regression after hyperopic PRK can manifest with or without corneal haze [19]. Pietilla et al., detected higher grades of corneal haze following PRK surgery in high grade hyperopic patients (>+6.00 D) [18]. A study by Jackson et al., reported lower haze rates in low-medium grade hyperopia groups compared to high grade hyperopia group [15]. In myopic PRK, subepitheal haze occurs in central cornea reaching maximum levels in 3-6 months [21-25]. In hyperopia ablation was performed from the peripheral cornea. In presented studies, circular haze diameter was measured as 6.5 mm at 4th week, and maximized in 3-9 months. The density of haze was lowered in 12-24 months. Peripheral haze stays persistent in eyes with higher dioptric correction [18-26]. In our study, we detected corneal haze in 6.57% of patients in group 1 and 31.57% of patients in group 2. Higher grades of corneal haze were found in patients with higher hyperopic refractive errors (group 2). Density of haze was highest between 1 and 3 months and subsided at 12th month postoperatively. Our study revealed that higher haze rates might be seen in eyes with higher hyperopic corrections.
The reliability of the hyperopic PRK treatment has been assessed by using BCVA levels following the surgery. Various reports on the reliability of this procedure have been published. A study by O’Brart et al., demonstrated that after 7.5 year follow-up period in eyes that received hyperopic PRK treatment, 65% had stable or increased BCVA levels. However 30% had one line vision loss, and 5% had two lines vision loss during this period [22]. Pacella et al., revealed that 92.8% of eyes had stable or increased BCVA levels, while 25% of eyes had 2 or more lines of vision loss on 7th day of the hyperopic PRK treatment. Reliability index of PRK treatment in hyperopic eyes was calculated as 1.04 [9]. In our study, we did not detect any decrease in BCVA n low grade hyperopia group. However in high grade hyperopia group 6.45% eyes had one and 3.22% eyes had two Snellen lines decrease in their BCVA. We calculated the reliability index of PRK treatmentas 1.02 in low and 0.98 in high grade hyperopia groups.
Efficacy index is calculate as mean postoperative UCVA/mean preoperative BCVA. Pacella et al., found UCVA values in last control appointment as 20/20 or better in 13 eyes and 20/32 in all eyes. Efficacy index was calculated as 0.93 (9,22-26). In our study results, efficacy index was calculated as 0.92 in low grade hyperopia group and 0.79 in high grade hyperopia group.
Predictability is defined as having postoperative target refraction values between ± 0.50 and ± 1.00 D. O’Brart et al., revealed that in high grade hyperopia, 40% of the patients were within ± 1.00 D and 24% of the patients were within ± 0.50 D range. Predictability was found to be better in correction in low and medium grade hyperopia but lower in high grade hyperopia group. Those results are similar to the other studies previously published [18, 26-29]. Dausch et al., (68 hyperopic eyes, range +2.00 to +8.25 D) calculated the predictability value as 59% in ± 0.50 D and 81% in ± 1.00 D range after one year postoperatively [4]. In our study, 92.5% of the eyes in low grade hyperopia group and 85.6% in high grade hyperopia group were within ± 1.00 D range. Predictability within ± 0.50 D range was found to be 82.4% in low grade and 62.8% in high grade hyperopia groups. Those prediction values are quite good when compared to previously published studies in the literature.
Conclusion
In the light of our findings, PRK was found to be a reliable, effective and predictable method in correction of low and high grade hyperopia.
References
- Taneri S, Zieske JD, Azar DT. Evolution, Techniques, Clinical Outcomes, and Pathophysiology of LASEK: Review of the Literature. Surv Ophthalmol. 2004 Nov-Dec;49(6):576-602. PubMed PMID: 15530945.
- Pallikaris IG, Katsanevaki VJ, Kalyvianaki MI, Naoumidi II. Advances in subepithelial excimer refractive surgery techniques: Epi-LASIK. Curr Opin Ophthalmol. 2003 Aug;14(4):207-12. PubMed PMID: 12888719.
- Melki SA, Azar DT. LASIK complications: etiology, management, and prevention. Surv Ophthalmol. 2001 Sep-Oct;46(2):95-116. PubMed PMID: 12888719.
- Dausch D, Smecka Z, Klein R, Schröder E, Kirchner S. Excimer laser photorefractive keratectomy for hyperopia. Journal of Cataract & Refractive Surgery. 1997 Mar 1;23(2):169-76.
- Durrie DS, Schumer DJ, Cavanaugh TB. Holmium: YAG laser thermokeratoplasty for hyperopia. J Refractive Corneal Surg. 1994 Mar 1;10(2):S277- 80.
- Basuk WL, Zisman M, Waring GO III, et al. Complications of hexagonal keratotomy. Am J Ophthalmol. 1994 Jan 15;117(1):37-49. PubMed PMID: 8291591.
- Epstein D, Fagerholm P, Ramberg-Nystrom H, Tengroth B. Twenty-fourmonth follow-up of excimer laser photorefractive keratectomy for myopia: Refractive and visual acuity results. Ophthalmology. 1994 Sep;101(9):1558-63; discussion 1563-4. PubMed PMID: 8090458.
- Snibson GR, Carson CA, Aldred GF, Taylor HR. One-year evaluation of excimer laser photorefractive keratectomy formyopia and myopic astigmatism. Melbourne Excimer Laser Group. Arch Ophthalmol. 1995 Aug;113(8):994-1000. PubMed PMID: 7639676.
- Pacella E, Abdolrahimzadeh S, Giorgi D, et al. Excimer laser photorefractive keratectomy for hyperopia and hyperopia astigmatism. Invest Ophthalmol Vis Sci. (ARVO) 1998;40(4):4114:781.
- Jackson WB, Mintsioulis G, Agapitos PJ, et al. Excimer laser photorefractive keratectomy for low hyperopia: safety and efficacy. J Cataract Refract Surg. 1997 May;23(4):480-7. PubMed PMID: 9209981.
- O'Brart DP, Stephenson CG, Oliver K, et al. Excimer laser photorefractive keratectomy for the correction of hyperopia using an erodible mask and axicon system. Ophthalmology. 1997 Nov;104(11):1959-70. PubMed PMID: 9373133.
- Brancato R, Carones F, Morico A, et al. Hyperopia correction using an erodible mask excimer laser delivery system coupled to an axicon:Preliminary results. Eur J Ophthalmolgy. 1997 Jul-Sep;7(3):203-10. PubMed PMID: 9352271.
- Sener B, Ozdamcar A, Aras C, et al. Photorefractive keratectomy for hyperopia and aphakia with a scanning spot excimer laser. J RefractSurg. 1997 Nov-Dec;13(7):620-3. PubMed PMID: 9427199.
- Vinciguerra P, Epstein D, Radice P, et al. Long-term results of photorefractive keratectomy for hyperopia and hyperopic astigmatism. J Refract Surg. 1998 Apr;14(2 Suppl):S183-5. PubMed PMID: 9571549.
- Jackson WB, Casson E, Hodge WG, et al. Laser vision correctionfor low hyperopia. An 18-month assessment of safety and efficacy. Ophthalmology. 1998 Sep;105(9):1727-38; discussion 1737-8. PubMed PMID: 9754184.
- Carones F, Brancato R, Morico A, et al. Photorefractive keratectomy for hyperopia using an erodible disc and axicon lens: 2-year results. J Refract Surg. 1998 Sep-Oct;14(5):504-11. PubMed PMID: 9791816.
- Corones F, Gobbi PG, Vigo L, et al. Photorefractive keratectomy for hyperopia: Long-term nonlinear and vector analysis of refractive outcome1. Ophthalmology. 1999 Oct 1;106(10):1976-83.
- Pietila J, Makinen P, Pajari S, et al. Excimer laser photorefractive keratectomy for hyperopia. J Refract Surg. 1997;13:504-510. PubMed PMID: 9352478.
- Reinstein DZ, Silverman RH, Sutton HF, et al. Very high-frequency ultrasound corneal analysis identifies anatomic correlates of optical complications of lamellar refractive surgery: Anatomic diagnosis in lamellar surgery. Ophthalmology 1999 Mar;106(3):474-82. PubMed PMID: 10080202.
- Hosoda Y, Nakayasu K. [A confocal microscopic and histological study on rabbit corneas after photorefractive keratectomy for hyperopia. Nippon Ganka Gakkai Zasshi. 1999 Mar;103(3):243-51. PubMed PMID: 10214060.
- Dierick HG, Van Mellaert CE, Missotteni L. Histology of rabbit corneas after 10-diopter photorefractive keratectomy for hyperopia. J Refract Surg. 1999 Jul 1;15(4):459-68.
- O'Brart DP, Patsoura E, Jaycock P, et al. Excimer laser photorefractive keratectomy for hyperopia: 7.5-year follow-up. J Cataract Refract Surg. 2005 Jun;31(6):1104-13. PubMed PMID: 16039483.
- El-Agha MS, Johnston EW, Bowman RW, et al. Excimer laser treatment of spherical hyperopia: PRK or LASIK? Tr Aml Ophth Soc. 2000;98:59-69.
- Salz JJ, Maguen E, Nesburn AB, et al. A two-year experience with excimer laser photorefractive keratectomy for myopia. Ophthalmology 1993 Jun;100(6):873-82. PubMed PMID: 8510900.
- Tengroth B, Epsterin D, Fagerholm P, et al. Excimer laser photorefractive keratectomy for myopia; clinical results in sighted eyes. Ophthalmology 1993 May;100(5):739-45. PubMed PMID: 8493018.
- Tabbara KF, El-Sheikh HF, Islam SM. Laser in situ keratomileusis for the correction of hyperopia from +0.50 to +11.50 diopters with the Keracor 117C laser. J Refract Surg. 2001 Mar-Apr;17(2):123-8. PubMed PMID: 11310761.
- Stephenson CG, Gartry DS, O’Brart DP, et al. Photorefractive keratectomy; A 6-year follow-up study. Ophthalmology 1998 Feb;105(2):273-81. Pub- Med PMID: 9479287.
- O’Brart DP, Stephenson CG, Baldwin H, et al., Hyperopiclaser photorefractive keratectomy with the erodiblemask and Axicon system: two year followup. J Cataract Refract Surg. 2000; 26:524-535. PubMed PMID: 10771225.
- Dausch D, Klein R, Schro¨der E. Excimer laser photorefractive keratectomy for hyperopia. Refract Corneal Surg. 1993 Jan 1;9:20-28.