In Office Occlusion of the Tear Punctum using the CPO Electrode
Raus PPM1*,Verhaert PDEM2
1 Miró Center for Ophthalmology and Oculoplastic Surgery, Geel, Belgium.
2 Pathology Unity, General Hospital Herentals (AZ Herentals), Herentals, Belgium.
*Corresponding Author
Peter PM Raus,
Miró Center for Ophthalmology and Oculoplastic Surgery, Geel, Belgium.
Tel: 9418723653
E-mail: peter.raus@gmail.com
Received: January 30, 2017; Accepted: March 11, 2017; Published: March 15, 2017
Citation: Raus PPM, Verhaert PDEM (2017) In office Occlusion of the Tear punctum using the CPO Electrode. Int J Ophthalmol Eye Res. 5(3), 283-286. doi: dx.doi.org/10.19070/2332-290X-1700061
Copyright: Raus PPM© 2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Dry eye syndrome (DES) is a relatively common condition with signs and symptoms varying from light irritation to functional blindness as a result of corneal opacification. The disorder generally occurs in subjects over 40 years of age. DES management is related to the severity of the symptoms. In most cases treatment focuses on tear substitution.
However, permanent punctal occlusion may be indicated for patients in which these conventional therapies fail. The procedure is simple and can be performed in the office. To cauterize and close the punctum, a high-frequency low-temperature surgical device and a specifically designed electrode [Chedly Punctal Occluder (CPO)] are employed. The very same CPO electrode, may be utilized to achieve dilation of the punctum and sealing of the the lumen of the lacrimal duct.
In cases where a permanent punctal occlusion is indicated, the CPO, in combination with a high frequency device, permanently seals the punctum. In ost cases a scar is only visible by close slit lamp examination.
So far no cases of punctum reopening have been observed.
Although permanent punctal occlusion in DES is not the first treatment of choice, it sometimes has to be considered in severe DES cases, where other therapies remain unsuccessful.
2.Introduction
3.DES Diagnosis
4.Surgical Anatomy of the Tear Duct
5.Treatment in DES
6.Technique to Seal the punctum
7.Results
8.Conclusion
9.Acknowledgement
9.References
Keywords
Dry Eyes; Punctal Occlusion; Radio Frequency Surgery; CPO.
Introduction
Dry eye disease (dry eye syndrome, DES) arguably is the most frequently encountered ocular disorder in our daily ophthalmological practice. The prevalence varies between 5.5% [1] and 33.7 % [2].
Yeta correct diagnosis appears to be extremely difficult and no single test is known to achieve this [3].
In 1995 the National Eye Institute of the USA proposed two main categories of ‘dry eyes’ and this division is still applied [4]:
• ‘Aqueous tear deficiency’, characterized by insufficient secretion of tears.
• ‘Evaporative dry eyes’ attributable to abnormally fast evaporation of tears.
Ten years later, a first classification for practical and clinical use was published by Murube et al., [5]. The International Dry Eye Workshop (DEWS) updated the definition and classification of dry eyes in 2007 [6]:
‘Dry eye is a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance and tear film instability with potential damage to the ocular surface. It is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface’
A common symptom in both aqueous tear deficiency and evaporative dry eyes is increased tear osmolarity, and hence osmolarity of tears is considered a valuable parameter for clinical diagnosis and evaluation the degree of dry eyes [7].
As such, assessing the degree of dry eyes is an important and decisive factor in determining the DES patient’s therapy. Until a superior cure for dry eye disease will be discovered, therapeutic treatments limited to:
1. Try and raise the quantity of tears.
2. To prevent the loss or evacuation of tears.
Examples to raise the quantity of tears are:
• Substitution therapy with artificial tears or gels,
• Stimulation of secretion of tears or components of tears (e.g., Rebamipide, Diquafosol, cholinergic agonists), or
• Transplantation of labial salivary glands to the eyelids [10].
Examples to limit tear evacuation are:
• Induction of ptosis,
• Lateral or medial tarsorrhaphy
• Botulinum toxin injection around the inferior punctum,
• Obstruction of one or more puncta lacrimalia by inserting a silicone plug [8, 9], or
• The use of the ‘Chedly punctal Occluder’ (CPO), developed by Bouzouaya to coagulate a punctum [8].
DES Diagnosis
Many test are reported to evaluate the quality and quantity of tears but not all of them are usable in the office to assess cause and degree of our patients’ dry eyes. For research purposes we are analyzing the protein composition of tears in healthy and dry eye patients by mass spectrometry. The underlying idea is that this could yield biomarkers to assist in a correct differential diagnosis for the many different forms of dry eyes [19-21]. In addition, we anticipate that comparing the proteins in tears of dry eye patients with those of healthy subjects will yield invaluable information about DES pathophysiology and consequently suggestions for a better or more personalized therapy [11]. In the office the most frequently used tests are:
• Evaluation of the tear meniscus height: less than 1mm thick is considered as suggestive for DES.
• The tear break-up time (BUT) with fluorescein: has to be higher than 10 sec (the tear BUT is the time measured from the blink to the appearance of tear film defects).
• Schirmer’s test without topical anaesthesia indicates basal and reflex tearing (the norm usually used is more than 15mm at 5 minutes).
• Schirmer’s test with topical anaesthesia measures basal tearing only (the norm is: greater than 10mm at 5 minutes).
Surgical Anatomy of the Tear Duct
Tears enter the lacrimal drainage system through the upper and lower puncta lacrimalia of the eyelids. They are located on the medial lid margin. The papillae appear pale in contrast to the surrounding tissue because they contain more connective tissue and are less vascularized. They are centered on the eyelid margin in line with the mucocutaneous junction. The upper papillae are located approximately 6mm from the medial canthal angle and the lower papillae are 6.5mm from the medial canthus. The punctal opening measures 0.2-0.3mm in diameter and is surrounded by a ring of dense connective tissue that normally maintains a patent entrance. In youth the aperture is round or oval, whereas with age, this often collapses into a slit, which typically delays normal evacuation of tears. The puncta are normally directed some what posterior towards the globe and do not become visible unless the lid is slightly everted. As soon as the punctum is visible, we already call it ectropion. From each punctum, a canaliculus passes perpendicular to the lid margin at a distance of 2mm, where it dilates to form the ampulla, an irregular sac 2mm in diameter. From the ampulla, the canaliculi turn horizontally and run medially, parallel to the lid margin for a distance of approximately 8mm. In 90% of individuals, the two canaliculi join at an angle of approximately 25 degrees to form a common canaliculus 3-5mm in length. In the remaining 10%, the two join the lacrimal sac independently [12, 13].
The lacrimal sac passes inferiorly into a bony canal of 12mm in length and 3-5mm in width. From the lacrimal sac the lacrimal duct goes down to the nose where it ends below the inferior nasal concha.
Treatment in DES
Since in the majority of cases a causal therapy is not possible yet, the treatment will be limited to a substitution of normal tears or an attempt to limit the consequences of the dry eye.
Generally speaking, the treatment can be divided into 5 groups:
• Substitution of normal tears (e.g. artificial tears, gels,…),
• Stimulation of secretion of tears or tear components (e.g. cholinergic agents, Diquafosol, Rebamipide,…),
• Prevention of evaporation or outflow of tears by, e.g.,punctal plugs, coagulation of the tear puncta),
• In cases of severe dry eyes, symptomatic treatmentwith anti-inflammatory drugs…),
• Avoidance and/or reduction of additional factors triggering dry eyes (e.g. dry air, computer work, …).
In this paper we focus on the prevention of tear outflow by using punctal plugs versus coagulation of the puncta.
Although at first sight the prevention of outflow of tears seems to be an elegant solution to increase the quantity of tears, the technique has is limitations:
• Reduced tear outflow also slows down the elimination of microorganisms and allergens. Especially in younger patients this can cause bacterial or allergic conjunctivitis.
• In older patients the secretion of tears might be so limited that obstruction of the tear outflow has only negligible effect.
• In DES patients without a known cause, the problem might be temporary; in such cases it is safer to opt for a reversible obstruction of the tear duct.
In case of doubt a soluble collagen plug can be implanted in one lower punctum. In the hours after implantation the plug will swell and obstruct the tear duct for about one week, the time for the plug to be dissolved. If the plug relieves the symptoms of the patient to an acceptable level, a silicone plug can be implanted.
One should always choose for the smallest diameter of plug to close the punctum. If the plug must be taken out afterwards or pops out spontaneously, a thick plug will have dilated the punctum and the dilated punctum will aggravate the problem by increasing the outflow of tears through a dilated punctum. Some of the drawbacks of punctal plugs are migration into the nasolacrimal system, which can cause canaliculus and lacrimal sac inflammation and recurrent extrusion [7, 8]. In those cases and with a patient with severe dry eyes permanent occlusion of the tear duct is to be considered. Several techniques for permanent occlusion have been described.
Potentially reversible methods mostly involve mobilization or transfer of tissue with the possibility to reverse all or parts of the made changes. Examples are:
• Transfer of the punctum towards the external lid margin [14]
• Punctaltarsoraphy [15]
• Punctal patchwith a pedunculated flap of adjacent tarsal conjunctiva [14]
• punctum switch graft [16]
Irreversible methods mostly use heat-induced damage or shrinkage of tissue. For this coagulation cautery probes, diathermy and laser can be used to occlude the tear duct by heat-induced damage to the canalicular and pericanalicular tissues. The more lateral heat spread to the pericanalicular tissues the less the chance that the technique used is reversible. On the other side, not all techniques are as effective to occlude the tear duct. The efficacy of permanent closure depends on the technique used and is limited to 7% when only the vertical portion of the canaliculus is cauterized [17].
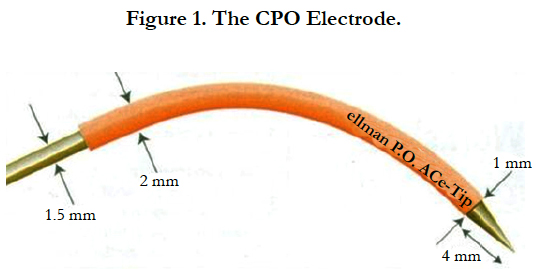
Also an Argon laser can be used to co close the punctum. This technique is known to give less postoperative discomfort, but the risk of recanalization appears to be significantly greater [18]. Moreover, the high cost of a laser may be a limiting factor to decide for this kind of treatment. That is why we prefer (and exclusively use) the high-frequency, low-temperature Surgitron® Dual radio frequency (RF) device (Ellman International Inc.) for this type of intervention. The RF device is more cost-effective than the laser therapy and has only limited side-effects in comparison with thermal cautery. With the Surgitron®, which we use for eyelid interventions, we prefer the CPO electrode specifically designed to cauterize and close the punctum by ChedlyBouzouaya and developed by Jon Garito (Figure 1). The CPO, made of tungsten, is designed to both dilate the punctum and to be inserted in the lumen of also the horizontal portion of the lacrimal duct. The CPO is the coneshaped Ellman RF electrode TNAEE287, patented and approved by the US Food and Drug Administration (FDA). Because it also gently coagulates the lateral part of the horizontal canaliculus, the CPO is very effective in permanently closing the tear duct in DES patients with only one brief in office treatment. The electrode is autoclavable and thus re-usable.
Technique to Seal thepunctum
The lower eyelid is infiltrated in the nasal side with Xylocaine 2% with epinephrine, first on the external side and then on the conjunctival side. Because the tip of the CPO is cone-shaped, no dilation of the punctum is required.
The CPO is mounted on the handpiece of radiosurgical unit that is put in a cut/coagulation mode. Only the non coated, metallic tip of the electrode is inserted in the canaliculus. Then the unit is activated for a few seconds while the CPO is slowly withdrawn. If the punctum is still visible, the tip of the CPO is applied on the lumen to coagulate and seal it [8]. An antibiotic ointment can be applied once or twice a day for a few days. The patient is seen after one week for a check-up. No patch is necessary. Because the procedure is easy, it can be performed in the office.
Results
During the last 16 months 11 inferior puncta were coagulated with the CPO;
Indications for permanent occlusion were:
• Recurrent loss of silicone plugs: 8 cases,
• Granuloma around punctalplug :1 case,
• Severe dry eye with excellent results after implantation of temporary collagen plug: 2 cases.
All patients were over 60 years old and had severe dry eyes with Schirmer’s test + topical anaesthesia < 5 mm and insufficient response to artificial tears application of 6 or more times daily.
The result was excellent in 8 cases. In two cases, where a perfect occlusion of the punctum was achieved, the patient did not report subjective improvement. Schirmer’s test was about the same. In one case the punctum re-opened a few weeks after coagulation. In that patient the canaliculus was cut and the stumps were coagulated. The wound was closed with three sutures of Vicryl 6/0. This led to an acceptable but limited improvement.
Conclusion
Despite the limited indications for permanent occlusion of the punctum lacrimale, the CPO appears to be a valuable instrument in our daily practice. When used for the right indications, coagulation of the lacrimal punctum using the CPO, in combination with a high frequency low-temperature radiofrequency device, yields excellent results. The therapy can be performed in the office and is very cost-effective when compared to a laser coagulation.
Acknowledgement
The authors gratefully acknowledge support by Z. de Silva (Melbourne, Australia) and Allergan (Hoeilaart, Belgium).
References
- Mc Carty CA, Bansal AK, Livingston PM, Stanislavsky YL, Taylor HR (1998) The epidemiology of dry eye in Melbourne, Australia. Ophthalmol. 105(6): 1114-1119.
- Lin PY, Tsai SY, Cheng CY, Liu JH, Chou P, et al., (2003) Prevalence of dry eye among an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmol. 110(6): 1096-1101.
- Savini G, Prabhawasat P, Kojima T, Grueterich M, Espana E, Goto E (2008) The challenge of dry eye diagnosis. Clin Ophthalmol. 2(1): 31–55.
- Lemp MA (1995) Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 21: 221-232.
- Murube J, Németh J, Höh H, Kaynak-Hekimhan P, Horwath-Winter J, et al., (2005) The triple classification of dry eye for practical clinical use. Eur J Ophthalmol. 15(6): 660-667.
- Report of the 2007 International Dry Eye Workshop. Ocul Surf. 5: 65-204.
- Lemp MA, Bron AJ, Baudouin C, Benítez Del Castillo JM, Geffen D, et al., (2011) Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 151(5): 792-798.
- Bouzouaya C, Raus P (2012) The Chedly punctal occluder: A new treatment modality for dry eyes. PRIME. 2: 30-37.
- Ervin AM, Wojciechowski R, Schein O (2010) Punctal occlusion for dry eye syndrome. Cochrane Database Syst Rev. 9: CD006775.
- Geerling G, Raus P, Murube J (2008) Minor salivary gland transplantation. Dev Ophthalmol. 41: 243-2.
- Raus P, Kumar Raghumaran B, Pinkse M, Verhaert P (2015) Bottom–up protein identifications from microliter quantities of individual human tear samples. Important steps towards clinical relevance. EuPA Open Proteomics. 9: 8-13.
- Paulsen F (2008) Anatomy and physiology of efferent tear ducts. Ophthalmologe. 105(4): 339-345.
- Tucker NA, Tucker SM, Linberg JV(1996) The anatomy of the common canaliculus. Arch Ophthalmol. 114(10): 1231-1234.
- Murube-del-Castillo J, Hernandez-King J (1993) Treatment of dry eye by moving the lacrimal punctum to dry dock. Ophthalmic Surg. 24(1): 53-58.
- MurubeJ, Murube E (1996) Treatment of dry eye by blocking the lacrimal canaliculi. Surv Ophthalmol. 40(6): 463-480.
- Geerling G, Tost FHW (2008) Surgical Occlusion of the Lacrimal Drainage System. Dev Ophthalmol. 41: 213-229.
- Knapp ME, Frueh BR, Nelson CC, Musch DC (1989) A comparison of two methods of punctalocclusion. Am J Ophthalmol. 108(3): 315-318.
- Benson DR, Hemmady PB, Snyder RW (1992) Efficacy of laser punctual occlusion. Ophthalmol. 99(4): 618-621.
- Zhou L, Beuerman RW, Chan CM, Zhao SZ, Li XR, et al., (2009) Identification of tear fluid biomarkers in dry eye syndrome using iTRAQ quantitative proteomics. J Proteome Res. 8(11): 4889-4905.
- Tong L, Zhou L, Beuerman RW, Zhao SZ, Li XR (2011) Association of tear proteins with Meibomian gland disease and dry eye symptoms. Br J Ophthalmol. 95(6): 848-852.
- Zhou L, Zhao SZ, Koh SK, Chen L, Vaz C, et al., (2012) In-depth analysis of the human tearproteome. J Proteomics. 75(13): 3877-3885.