Tibial Periosteum For The Surgical Perforation
Mateo A1, Rodrigo MJ1,2*, Abecia E1, Idoipe M1,2, Hualde A3, Satue M1,2
1 Ophthalmology Department, Miguel Servet University Hospital, Zaragoza, Spain.
2 Aragones Institute of Health Sciences, Zaragoza, Spain.
3 Traumatology Department, Miguel Servet University Hospital, Zaragoza, Spain.
*Corresponding Author
Maria Jesus Rodrigo MD,
Ophthalmology Department,
Miguel Servet University Hospital,
Zaragoza, 50009, Spain.
Tel: 0034.976.76.55.58
E-mail: mariajesusrodrigo@hotmail.es
Received: November 18, 2016; Accepted: Decmber 22, 2016; Published: December 23, 2016
Citation: Mateo A, Rodrigo MJ, Abecia E, Idoipe M, Hualde A, et al., (2016) Tibial Periosteum For The Surgical Perforation. Int J Ophthalmol Eye Res. 4(11), 266-269. doi: dx.doi.org/10.19070/2332-290X-1600056
Copyright: Rodrigo MJ© 2016. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Purpose: Report a successful case of scleral perforation repair, refractive to treatment with bank-scleral graft, using pretibial
periosteum graft patch.
Case Report: A patient who suffered a traumatic scleral perforation was treated unsuccessfully with bank-scleral patch graft. An autologous pretibial periosteal patch graft was then obtained and sutured to the scleral rupture with the osteogenic layer facing the sclera. The periosteum patch was covered by amniotic membrane and conjunctiva.
Results: Early vascularization was observed in the first seven days postoperative. The autologous periosteal patch graft and conjunctiva remained stable over a follow-up period of 6 months.
Conclusion: An autologous periosteal patch graft could be a good alternative after a non-successful bank-scleral patch repair of a scleral perforation.
2.Introduction
3.Case Report
4.Results
5.Discussion
6.References
Keywords
Scleral Reparation; Perforation; Periosteum.
Introduction
Scleral perforation, that is the disruption in the protective outer layer of the eyeball, is a severe situation that may cause important complications such as ocular pain, red eye, infections, low visual acuity, hypotonia, and choroidal or retinal detachment [1].
Anti-inflammatory and antibiotic medical treatment is used to avoid inflammation and infection. Surgical treatment consists of maintaining the tightness of the eyeball. Patchs made of different materials may be used for grafting, with sclera as the most commonly used tissue for the surgical repair of scleral perforation. However, several authors have cited the failure of scleral grafts due to melting (caused by the absence of vascularization) or to immunomediated rejection [2].
Fascia lata, split-thickness dermal graft, or periosteum graft may also be used with optimal results [2].
Periosteum is a specialized tissue covering the bone surface. It consists of an inner osteogenic layer and an outer layer of dense connective tissue, made of fibroblasts, collagen and dense vascularization [3].
Using autogenous periosteum as a graft has several advantages: harvesting and vascularization is easy [3], it has an excellent tensile strength, provides ample tectile support, it is not susceptible to immunological rejection and it is easily available [4]. On the other hand, there are some disadvantages: periosteum provides an irregular surface, thus covering the graft with a conjunctival flap is needed [3]. Eventually, the periosteum graft could develop bone formation, as a late complication due to the osteogenic layer [5].
We present the case of a patient who received autologous periosteum as a graft to repair a traumatic scleral perforation. There are very few published studies on the use of periosteum as a graft for the surgical repair of this severe condition [2, 3, 5].
Case Report
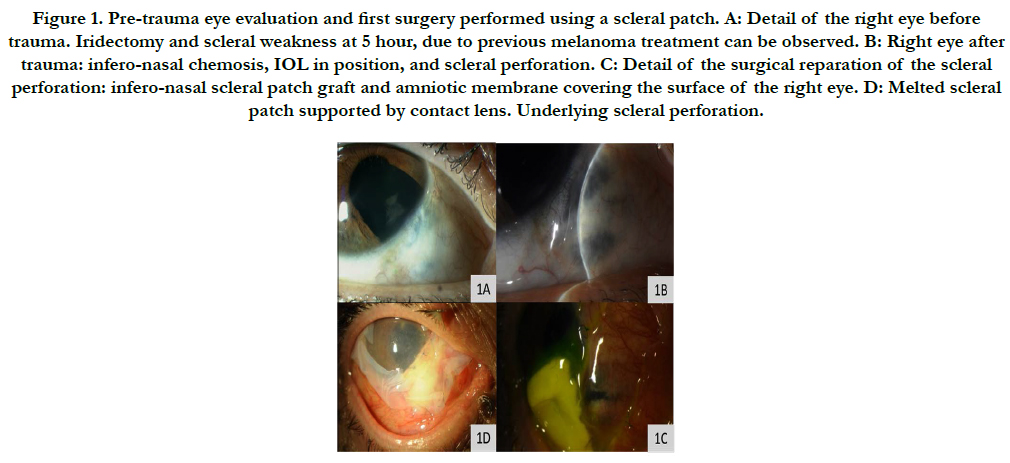
A 57 -year- old Caucasian female, suffered a scleral perforation in her right eye (OD) due to a low energy trauma with a plastic container. She had a previous history of iridian and ciliary body melanoma in her OD treated with both surgery and radiotherapy, thirteen years ago and was controlled periodically for scleral weakness [Figure 1A].
After trauma, the ophthalmic exam revealed infero-nasal bullous chemosis in her OD, haematic Tyndall, ocular hypotonia and vitreous hemorrhage [Figure 1B]. Although the intraocular lens (IOL) was not displaced, her visual acuity was light perception.
The patient was prepared for surgical reparation under general anesthesia, prior oral corticoid treatment (metilprednisolone, 70 mg). A 10 x 7 mm harvested scleral patch was sutured to healthy sclera, covering the default. Amniotic membrane (AM) was then attached to the surface of the scleral patch, using biological tissue adhesive (Tissucol Duo®, Baxter AG, Viena, Austria). An 8x8 mm autologous conjunctival graft was placed over the AM patch and sutured with 10-0 nylon plus biological adhesive. A second patch of AM was sutured to the surface of the eye to help reduce inflammatory reaction. A bandage contact lens was used to protect the cornea surface [Figure 1C].
The patient was prescribed oral ciprofloxacine (500 mg twice per day), omeprazol and taper treatment with oral prednisone (starting 60 mg per day). Topic prednisolone acetate, moxifloxacin and cyclopentolate drops 3 times daily were also prescribed.
During the early postoperative (first 7 days) the scleral graft presented melting. Thus, it had to be removed [Figure 1D].
Figure 1. Pre-trauma eye evaluation and first surgery performed using a scleral patch. A: Detail of the right eye before trauma. Iridectomy and scleral weakness at 5 hour, due to previous melanoma treatment can be observed. B: Right eye after trauma: infero-nasal chemosis, IOL in position, and scleral perforation. C: Detail of the surgical reparation of the scleral perforation: infero-nasal scleral patch graft and amniotic membrane covering the surface of the right eye. D: Melted scleral patch supported by contact lens. Underlying scleral perforation.
The patient was evaluated once a week for 10 weeks by biomicroscopy, Goldman intraocular pressure (IOP), anterior segment photography (Nikon d90 camera), and anterior and posterior segment Swept–source optical coherence tomography (SS-OCT) (Triton OCT, Topcon corporation, Japan).
The patient finally developed a progressive conjunctival cyst, negative Seidel, and macular oedema secondary to an epiretinal membrane. A combined surgery was planned.
A triple procedure was performed, consisting of periosteum extraction (carried out by an orthopedic surgeon), pars plana vitrectomy (PPV) and epiretinal membrane peeling (performed by a retina surgeon) and finally scleral reparation with periosteum graft (performed by a corneal surgeon).
All surgical steps were performed aseptically and under general anesthesia.
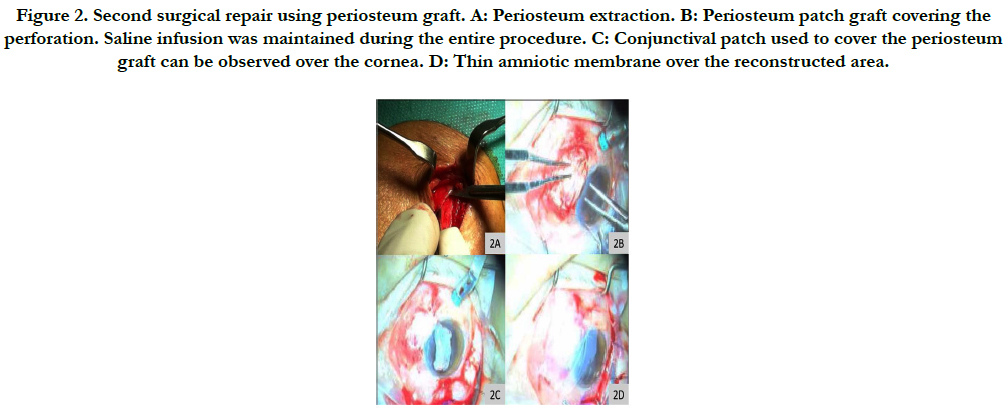
A proper-sized piece of periosteum was obtained from the anteromedial surface of the patient’s left tibia through a vertical incision. The muscles were bluntly dissected off the periosteum and a 10 x 8 mm graft was excised with scalpel and scissors [Figure 2A]. The periosteum graft was soaked in saline.
A 25 gauge PPV and epiretinal membrane peeling was performed with the usual procedure [1]. Saline continuous infussion was kept until the scleral reconstruction had concluded.
A 360º peritomy was performed, 6-7 millimeters from the scleral perforation. Healthy sclera was exposed. The periosteum graft was then placed with the osteogenic inner layer facing the sclera and sutured to the healthy tissue using interrupted sutures (8-0 nylon) [Figure 2B].
We placed the graft as found naturally on the bone: that is, the osteogenic inner layer in contact with the sclera, the outer connective layer facing the surface.
An autologous conjunctival patch obtained from the superior bulbar conjunctiva of her left eye was transplanted over the periosteum graft. AM and a bandage contact lens were placed over the graft to protect the ocular surface [Figure 2C and 2D].
Figure 2. Second surgical repair using periosteum graft. A: Periosteum extraction. B: Periosteum patch graft covering the perforation. Saline infusion was maintained during the entire procedure. C: Conjunctival patch used to cover the periosteum graft can be observed over the cornea. D: Thin amniotic membrane over the reconstructed area.
Results
Postoperative oral prednisone (30 mg twice per day), and ciprofloxacine (500 mg daily) were prescribed. Topic treatment with dexametasone-tobramicine drops 4 times per day, cyclopentolate and bromfenac drops twice per day, for 2 weeks was prescribed. The patient was evaluated every one, two or three weeks for a period of 12 weeks.
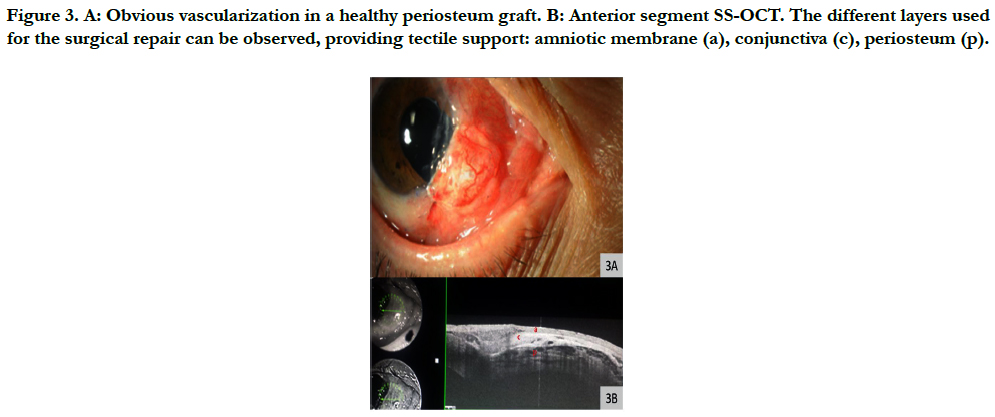
The periosteum graft provided adequate coverage of the scleral perforation and evident vascularization was observed 7 to 10 days postsurgery [Figure 3A and 3B]. There was no evidence of infection or graft rejection. The periosteal graft and conjunctiva remain stable 6 months after the procedure.
Figure 3. A: Obvious vascularization in a healthy periosteum graft. B: Anterior segment SS-OCT. The different layers used for the surgical repair can be observed, providing tectile support: amniotic membrane (a), conjunctiva (c), periosteum (p).
Discussion
In the current paper we describe an eye with traumatic scleral perforation treated with a periosteum graft.
There are very few scientific publications describing a scleral repair with periosteum graft [2, 3, 5], which is normally used when a scleral graft has failed.
In our patient, the first surgery performed with bank-sclera graft patch failed. Preoperative and postoperative corticosteroid treatment may have prevented vascularization and fibroblast proliferation producing eventual scleral melting, as Nguyen et al., [3] described.
Several grafts may be use in the surgical repair of a scleral perforation. The most common types are sclera, yugalmucosae, fascia lata or dermal graft. Others such as aortic tissue, expanded Polytetrafluoroethylene (PTFE), or Gore-Tex patch were refused because of their poor results [6].
In our case yugalmucosae and dermal graft were rejected since our patient needed an ample tectile support, that is, a strong graft.
The histological features of osteogenic and dense connective layers were the reason to choose a periosteum graft. Moreover, the unusual type VIII collagen (present in the donor [periosteum] and the recipient [sclera] tissues) [7] was probably causative of the strong integration and subsequent vascularization in the area.
Obvious vascularization was observed in our patient in the early postoperative (7-10 days) much earlier than Mauriello et al., [8] described after using a dermal graft. This early vascularization probably occurred because periosteum graft has its own large capillary net, larger than a dermal graft, and may have played a crucial role in the adherence and early nourishment of the sclera.
To the best of our knowledge the surgical technique performed in our patient, has not been described before. We placed the osteogenic layer facing the recipient´s bed, that is, in contact with the sclera (and over the remnants of the patient´s conjunctiva) and the connective tissue facing outwards, contrary to what Gupta et al., described [9]. Additionally we avoided the irregular surface, obtaining a more aesthetic result.
We decided not to open the scleral perforation completely. It is known that the most detrimental potential complication of a scleral perforation is the perforation of the choroid, with the subsequent ocular hemorrhage [3]. Thus, we decided to take extra caution in the preparation of the surgical bed and maintained the continuous infusion of saline during the complete surgical time. Thanks to the use of periosteum and a meticulous technique we obtained an anatomical, functional and aesthetic surgical repair of the scleral perforation.
In our experience, we consider periosteum to be a malleable, easy working, obtainable, and strong graft, ideal for the surgical repair of scleral perforations. Periosteum tissue provides ample tectile support and great vascularization, which makes it especially useful in cases of large perforations, scleral weakness, immunomediated scleral inflammation r shortage of scleral tissue.
References
- Wong SC, Lee TC, Heier JS (2014) 23-Gauge endoscopic vitrectomy. Dev Ophthalmol. 54: 108-19.
- Breslin CW, Katz JL, Kauman HE (1977) Surgical Management of necrotizing scleritis. Arch Ophthalmol. 95(11): 2038-2040.
- Nguyen QD, Foster CS (1999) Scleral patch graft in the management of necrotizing scleritis. Int Ophthalmol Clin. 39(1): 109-31.
- Mauriello JA, Fiore PM, Pokorny KS, Cinotti DJ (1988) Use of split-thickness dermal graft in the surgical treatment of corneal and scleral defects. Am J Ophthalmol. 105(3): 244-247.
- Carrol CP, Keates RH (1979) Bone formation in a periosteal graft. Arch ophthalmol. 97(5): 916.
- Huang WJ, Hu FR, Chang SW (1994) Clinicopathologic study of Gore- Tex patch graft in corneoscleral surgery. Cornea. 13: 82-6.
- Kapoor R, Sakai LY, Funk S, Roux E, Bornstein P, et al., (1988) Type VIII collagen has a restricted distribution in specialized extracellular matrices. J Cell Biol. 107(2): 721-730.
- Mauriello Jr JA, Pokorny K (1993) Use of split-thickness dermal grafts to repair corneal and scleral defects- a study of 10 patients. Br J of ophthalmol. 77(6): 327-331.
- Gupta S, Anand R, Diwan S, Gupta N (2014) Salvaging recurrent scleral buckle exposure with autologous periosteal patch graft. Retin Cases Brief Rep. 8(3): 178-182.