Self-Assembled Complex of Melamine and Alloxan
Dandala N1, Hai-Feng Ji2*
1 Institute for Micromanufacturing, Louisiana Tech University, Ruston, LA, USA.
2 Department of Chemistry, Drexel University, Philadelphia, PA, USA.
*Corresponding Author
Hai-Feng Ji,
Department of Chemistry, Drexel University,
Philadelphia, PA 19104, USA.
E-mail: hj56@drexel.edu
Received: January 06, 2019; Accepted: January 06, 2019; Published: January 06, 2019
Citation: Dandala N, Hai-Feng Ji. Self-Assembled Complex of Melamine and Alloxan. Int J Nano Stud Technol. 2019;8(1):132-134. DOI : dx.doi.org/10.19070/2167-8685-1900024
Copyright: Hai-Feng Ji© 2019. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
In this short communication we report the synthesis and characterization of self-assembled complexes that are hydrogenbonded between alloxan monohydrate and melamine. The complexes were characterized by using the scanning electron microscopy, the infrared spectroscopy, and thermal calorimetry.
2.Introduction
3.Experimental
4.Results and Discussion
4.1 Differential Scanning Calorimetry Results
5.Conclusion
6.References
Key Words
Melamine; Alloxan; Supramolecular System; Microwire; Crystal; Complex.
Introduction
Supramolecular chemistry has been receiving a great deal of attention due to the synthesis of structures with well-defined architectures via non-covalent interactions between individual components [1-6]. Hydrogen bonding along with self-assembling is responsible for the formation of important architectures like DNA and protein folding. Recently, we reported several supramolecular structures involving various non-covalent interactions like hydrogen bonding, pi-pi stacking, and chelation of metal ions [7-10]. Of these interactions, hydrogen bonding is pivotal and is greatly employed in the formation of artificial supramolecules. In this article we report the synthesis and characterization of self-assembled complexes between alloxan monohydrate and melamine via hydrogen bonding.
Experimental
Alloxan monohydrate 98% (CAS#2244-11-3) was purchased from ALFA AESAR chemicals and Melamine (CAS#108-71-1) was purchased from Eastman Chemical Co. A solution of alloxan monohydrate in water was added to a hot aqueous solution of melamine. The solution was evaporated at room temperature to yield a white colored solid with an unusual glossy surface. SEM analysis of the complex was carried out using a Hitachi S-4800 Scanning Electron Microscope. Vibrational spectra of the complex were recorded on a Nexus FTIR spectrophotometer. The thermal stability of the complexes has been obtained on a METTLER TOLEDO DSC 823 instrument.
Results and Discussion
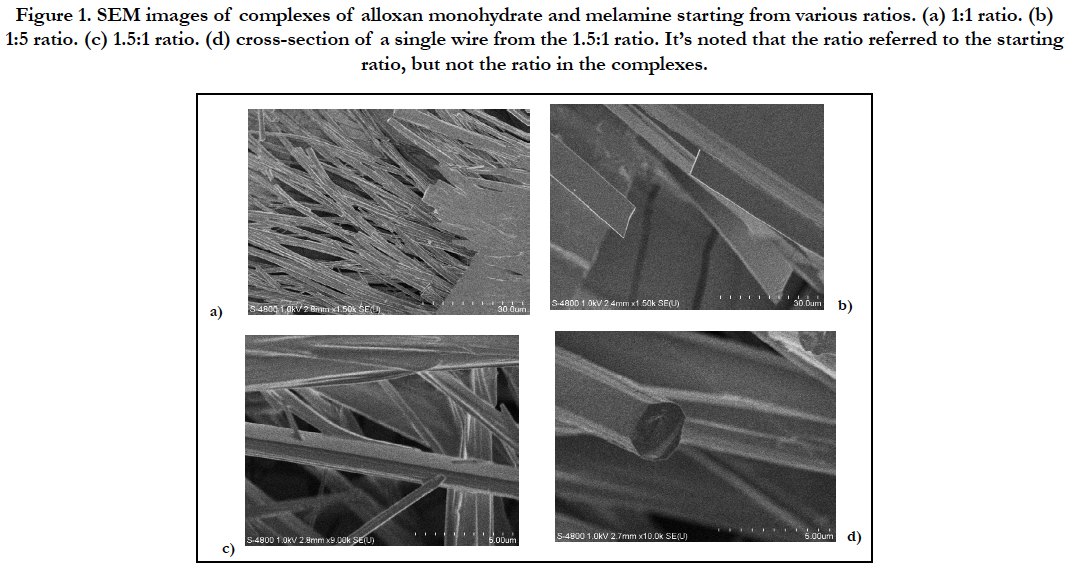
SEM analysis has been carried out on the complexes formed between alloxan monohydrate and melamine at different ratio. Complexes from 1:1 ratio and 5:1 ration show strips with the width ranging from 1.25 to 10 μm and the length extending to hundreds micrometers. The complex from 1.5:1 ratio seems to show microwires with a diameter of about 2-3 μm. The wires were sporadic, which made the isolation of wires very difficult.
Figure 1. SEM images of complexes of alloxan monohydrate and melamine starting from various ratios. (a) 1:1 ratio. (b) 1:5 ratio. (c) 1.5:1 ratio. (d) cross-section of a single wire from the 1.5:1 ratio. It’s noted that the ratio referred to the starting ratio, but not the ratio in the complexes.
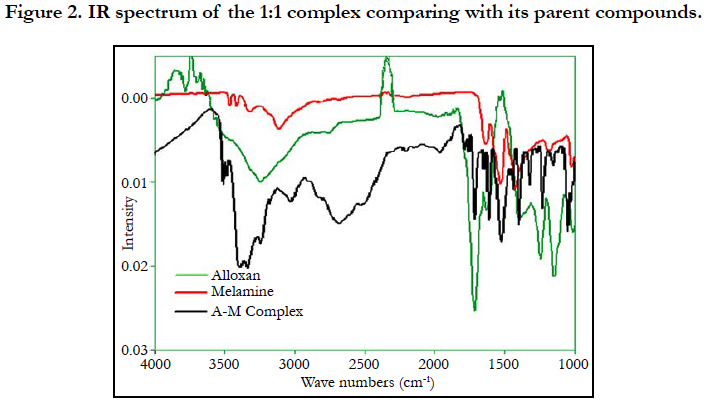
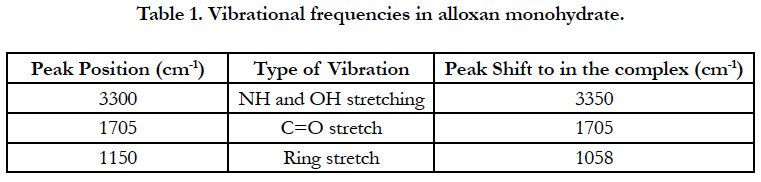
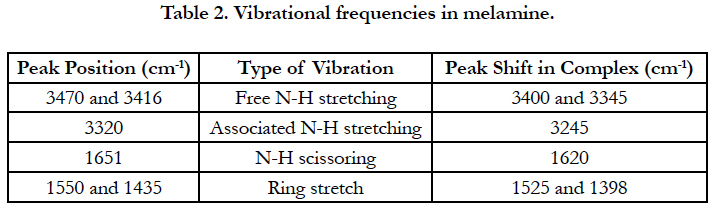
Figure 2 shows the IR absorption spectrum of a complex comparing to the IR spectra of the compounds melamine and alloxan monohydrate alone. The peaks and their attributions are listed in Tables 1 and 2 according to literatures [12, 13].
The major group in alloxan participating in the hydrogen bonding is the carbonyl group (C=O) at 1705 cm-1, However, no peak shift was observed, probably because C=O in alloxan alone was not free, but hydrogen-bonded with NH groups in other alloxan molecules. At around 3300 cm-1, a broad intense peak was observed, which is due to the hydrogen bonded NH and OH vibrations. This peak was shifted to around 3350 cm-1, which could contribute to new hydrogen bonded NH and OH vibrations in the melamine-alloxan complex.
In melamine, both 3470 cm-1 and 3416 cm-1 are due to the free asymmetric stretching –NH groups. They shifted to 3400 cm-1 and 3345 cm-1 in the complex. There was also a shift of 3320 cm-1 peak of melamine to 3245 cm-1. This corresponded to the associated symmetric stretching of -NH groups in melamine. The peak around 3400 cm-1 is board and intense, suggesting water molecules might also contribute to the infrared absorption in this area, i.e. water molecules were involved in the complex, which was confirmed by the thermal analysis. It is noteworthy that the FTIR spectra of complexes from other ratios were similar to that of the 1:1 ratio, suggesting a similar crystal structure, although the exact crystal structure is not solved yet due to the size of the microstructures.
Differential Scanning Calorimetry (DSC) of the complexes has been carried out for the complexes on a METTLER TOLEDO DSC 823 instrument. All the experiments were performed for a temperature range of 25°C to 600°C.
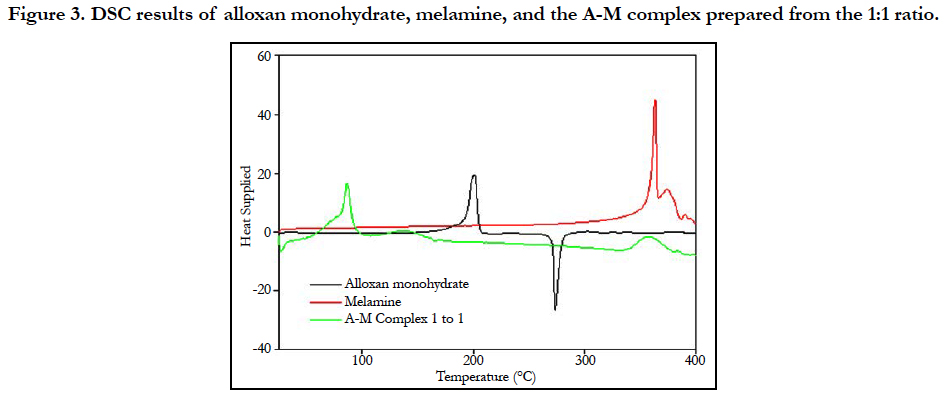
Figure 3 shows the DSC curves of alloxan monohydrate, melamine and the complex from the 1:1 ratio [14]. It has been reported that alloxan monohydrate melts when the temperature is greater than 250°C and then decomposes then. One interesting observation is that it loses water molecules incorporated into its structure at about 160°C. Melamine melts at 350°C and then it decomposes over a range of 100°C between 350 to 450°C. The endothermic peak at 350°C confirms this and the endothermic peak following is the conversion of melamine to melam.
Figure 3. DSC results of alloxan monohydrate, melamine, and the A-M complex prepared from the 1:1 ratio.
The DSC curve of the complex shows an endothermic peak at 85°C, which corresponds to the loss of water molecules. The temperature is lower than 100°C, suggesting these water molecules are freely trapped in the lattice but not coordinated in the network. Another endothermic peak is observed at 140°C which can be accounted to the loss of water which is coordinated in the complex. The complex melts and decomposes after 350°C. The peaks were not due to phase transitions since the peaks couldn’t be found in the cooling curves. The DSC of complexes from other ratios were similar to that of the 1:1 ratio, also suggesting a similar crystal structure for complexes prepared from different ratios.
Conclusion
Our preliminary study shows that alloxan and melamine form complexes when mixed in aqueous solutions. The crystal structure is under investigation, but the FTIR and DSC results suggest the presence of hydrogen bonding network between the two chemicals.
References
- MacGillivray LR, Atwood JL. A chiral spherical molecular assembly held together by 60 hydrogen bonds. Nature. 1997 Oct;389(6650):469.
- Seto CT, Whitesides GM. Synthesis, characterization, and thermodynamic analysis of a 1+ 1 self-assembling structure based on the cyanuric acid. cntdot. melamine lattice. J Am Chem Soc. 1993 Feb;115(4):1330-40.
- Seto CT, Whitesides GM. Molecular self-assembly through hydrogen bonding: supramolecular aggregates based on the cyanuric acid-melamine lattice. J Am Chem Soc. 1993 Feb;115(3):905-16.
- Reimschuessel R, Puschner B. Melamine toxicity-Stones vs. crystals. J Med Toxicol. 2010 Dec;6(4):468-9; discussion 470. doi: 10.1007/s13181-010- 0107-5. PubMed PMID: 20721654.
- Chang H, Wu G, Yue Z, Ma J, Qin Z. Melamine Poisoning Pediatric Urolithiasis Treatment in Gansu, China 5-Year Follow-up Analysis. Urology. 2017 Nov;109:153-158. doi: 10.1016/j.urology.2017.06.043. PubMed PMID: 28705579.
- Chen Y, Wang Q, Yan W, Tang H. Preparation of flame retardant polyamide 6 composite with melamine cyanurate nanoparticles in situ formed in extrusion process. Poly Degrad Stab. 2006 Nov 1;91(11):2632-43.
- Wang H, Kojtari A, Xu X, Ji HF. Self-Assembled Microwires of Terephthalic Acid and Melamine. Crystals. 2017 Jul 31;7(8):236.
- Ji HF, Samadi M, Gu H, Tomchak V, Qiao Z. Fabrication and applications of self-assembled nanopillars. AIMS Mate. Sci. 2017 Jul 19;4(4):905-19.
- Kojtari A, Ji HF. Metal organic framework micro/nanopillars of Cu (BTC)• 3H2O and Zn (ADC)• DMSO. Nanomaterials (Basel). 2015 Apr 9;5(2):565-576. doi: 10.3390/nano5020565. PubMed PMID: 28347026.
- Kojtari A, Carroll PJ, Ji HF. Metal organic framework (MOF) micro/nanopillars. Cryst Eng Comm. 2014;16(14):2885-8.
- Khopkar Y, Kojtari A, Swearer D, Zivanovic S, Ji HF. Perylenetetracarboxylic Diimide (PTCDI) Nanowires for Sensing Ethyl Acetate in Wine. J Nanosci Nanotechnol. 2014 Sep;14(9):6786-8. PubMed PMID: 25924331.
- Refat MS, El-Korashy SA, Kumar DN, Ahmed AS. Spectral and thermal studies of alloxan complexes. J Coord Chem. 2008 Jun 20;61(12):1935-50.
- Sayed MB. H-NMR, UV− Visible, and FT-IR Spectral Analyses for the Conflicting Impacts of Proton Mobility and H-Bonding Association on the Mesomeric Structure in Azopyrogallol, Catechol, Resorcinol, Quinol, and Phenol Derivatives of Melamine. Ind Eng Chem Res. 2004 Aug 4;43(16):4822-6.
- Qiu Y, Gao L. Blue-emitting cyanuric acid–melamine complexes from urea thermolysis. Mater Res Bull. 2005 May 18;40(5):794-9.