Structural and Chemical Research of Coordination Compounds of Hexaaqua Bisbenzol-1, 2, 4, 5- Tetracarbonate Diiron (II) With A Layered-Porous Structure, For Heavy Crude Oil
BT Usubaliyev1, DB Taghiyev2, VH Nurullayev3*, FB Aliyeva3, MK Munshiyeva2, PS Safarova3
1 Scientific-Research Institute "Geotechnological problems of oil, gas and chemistry", Azerbaijan State University of Oil & Industry, Baku, Azerbaijan.
2 Institute of Catalysis and Inorganic Chemistry after the name of M.F.Naghiyev, National Academy of Sciences of Azerbaijan, Baku, Azerbaijan.
3 Scientific-Research Institute "Geotechnological problems of oil, gas and chemistry", st. D.Aliyeva, Baku, Azerbaijan.
*Corresponding Author
VH Nurullayev,
Scientific-Research Institute "Geotechnological Problems of Oil, Gas and Chemistry",
AZ1010, st. D.Aliyeva, 227, Baku, Azerbaijan.
E-mail: veliehet1973@mail.ru
Received: January 02, 2017; Accepted: February 16, 2017; Published: February 20, 2017
Citation: BT Usubaliyev, DB Taghiyev, VH Nurullayev, FB Aliyeva, MK Munshiyeva, et al., (2017) Structural and Chemical Research of Coordination Compounds of Hexaaqua Bisbenzol-1, 2, 4, 5- Tetracarbonate Diiron (II) With A Layered-Porous Structure, For Heavy Crude Oil. 6(1), 123-127. DOI : dx.doi.org/10.19070/2167-8685-1700022
Copyright: VH Nurullaye© 2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
The complex compounds of diiron (II) bisbenzoll, 2, 4, 5 tetracarbonate were synthesized for the first time with porous structure. This article presents the results of X-ray diffraction, elemental and IR analyzes of spectroscopic derivatographic complex compound of 1,2,4,5-bis benzene tetracarbonate of diiron (II). By means of the indicated physical-chemical analyzes the individual coordination form of carboxyl groups, chemical formula and unit cell parameters of the complex compound have been identified. Also the process of thermal degradation of the studied complex has been studied. It was revealed that the compound after losing six molecules of water is resistant until the temperature 300°С. In all likelihood coordinately related the water molecules form intermolecular hydrogen bonds both with oxygen of the carboxyl groups, and among themselves. In all likelihood coordinately related the water molecules form intermolecular hydrogen bonds both with oxygen of the carboxyl groups, and among themselves. Here molecules of coordination water in the different layers play an active role in this interaction. Thus, a two-dimensional structure of a complex compound by means of the hydrogen bonds is transformed into 3D structure.
2.Introduction
3.Experimental Section
4.Synthesis of the Compound
5.Discussion of Results
5.1. X-ray analysis
5.2. Elemental Analysis
5.3. Thermal Decomposition
5.4. IR spectroscopic study
5.5. The Coordination Polyhedron is a Pyramid
6.Conclusions
7.Acknowledgements
8.References
Key Words
Complex Compound; A Layered Porous Structure; Pyromellitic Acid; Thermal Decomposition; Chemical Formula; IR Spectrum.
Introduction
Considerable interest to phthalates and terephthalates of various metals emerging recently is determined with their layered structure and the possibility of wide application. The layered structure of phthalates and terephthalates allows their use as molecular sieves and adsorbents, and particularly copper terephthalate is used for absorption of N2, Ar, Xe [1].
Tetraphtalate of zinc, calcium, and tin is used respectively as an activator of vulcanization in the rubber production, as a lubricant to prevent adhesion of rubber to technological fibers [2] as the capacitive dielectric [3] and terephthalate of ruthenium exhibits semiconductor properties [4, 5]. Also, we have synthesized a number of non-bonded compounds on the basis of the complexes of terephthalates of copper, cadmium (II) obtained in a slightly acid medium with formic acid [6], phthalates and terephthalates of cadmium, nickel (II) with acetic acid and phthalates and terephthalates of cadmium, cobalt, nickel and zinc (II) obtained in a basic medium (pH > 8.5) with acetic acid due to its layered structures [7-10].
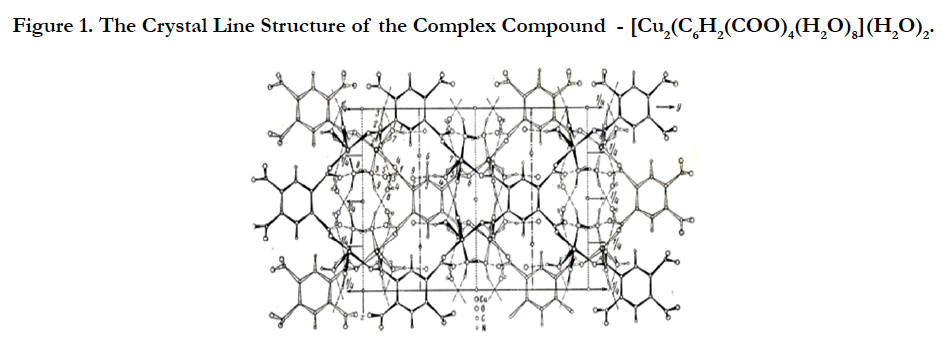
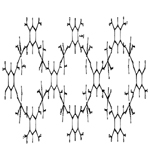
The complex compounds of copper (II) has been synthesized with benzene of 1, 2, 4, 5-tetracarboxylic acid (pyromellitic acid) and crystal-crystalline structure [11] have been decrypted (Figure 1).
As can be seen from the structure, it also has a polymer- laminate structure with pores of a certain size which are available for selfassembly. That is, a number of non-bonded compounds with various organic molecules of various sizes and geometries can be obtained on its bases.
For this purpose, on the basis of pyromellitic acid we obtained a number of complex compounds of various metals [12, 13].
This work presents the results of the synthesis, physicalchemical and structural studies of the chemical complex of hexaquabisbenzene 1, 2, 4, 5-tetracarbonate of diiron (II). Preliminary studies have shown that they improve the rheological properties of heavy oil, in particular, it produces the viscosity of heavy oil trade by 30%.
Experimental Section
The elemental composition of the obtained compound was determined by gas chromatography on an analyzer CHN30E Carlo ERBA. The metal content was calculated from the weight loss curve on quantity of oxide obtained after heating on derivatograph up to 800°С.
X-ray diffraction analysis was performed on the device Commander SampleID (Coupled Two Theta/Theta) WL 1.54060.
IR spectra were recorded on a device SPECORD-MBO in the area of 400-4000 sm-1.
Derivatograms were recorded on a derivatograph NETZSCH STA 449F3STA449F3A-0836-M (Range221/10.0(К/min)/800).
Synthesis of the Compound
The starting materials were C6H2(COOH)4(pyromellitic acid), FeCl2•4H2O, NaHCO3 of qualification Ch.p. (GOST 3759-75). Complex compound was obtained by reacting pyromellitic acid sodium salt with iron chloride (II) in a weakly acidic medium. During the reaction polycrystalline powder of the complex falls abundantly. Polycrystalline powder is heated up to boiling and kept boiling for 15 minutes. Then it was filtered while it was hot and washed for several times with distilled water. First it was dried on a filter paper at a room temperature and then in a drying oven at 50°С. The chemical formula of the complex connections was established on the basis of X-ray diffraction, elemental, TGA and IR spectroscopic analysis.
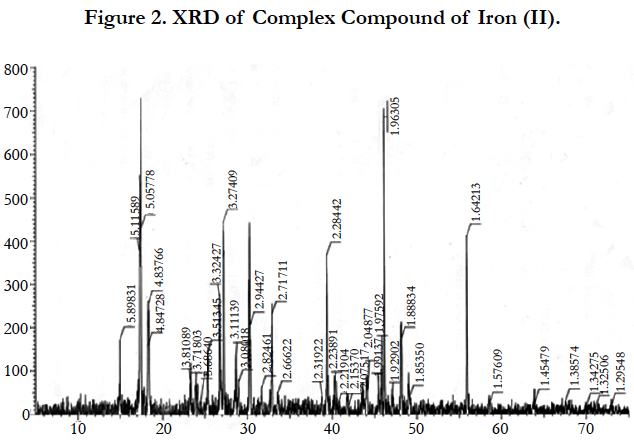
Figure 2 shows the X-ray of the complex compound. As seen from the radiographs, this compound is highly crystalline and has a high symmetry, as the peaks are located in all corners.
The parameters of the elementary cell of the complex compound have been determined with indexing diffraction patterns of: a = 10,1, b = 18,24, c =11,76Å.
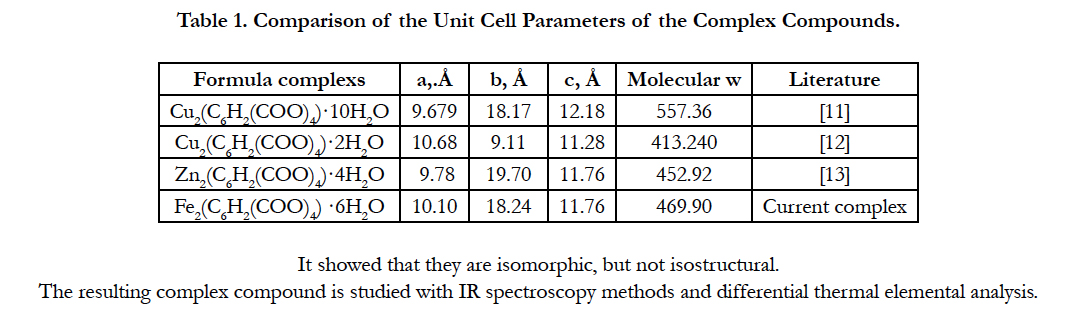
Comparison of the unit cell parameters of the current complex compound with the parameters of the known complexes (Table 1).
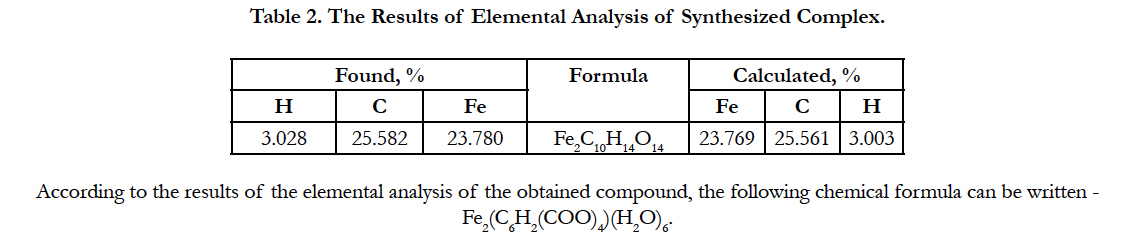
Elemental analysis results are presented in Table 2.
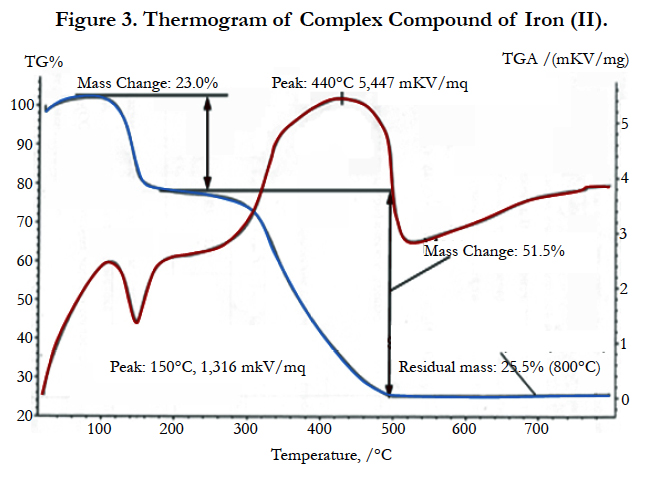
Thermogravigramma of the complex compound of the iron (II) is shown in Figure 3.
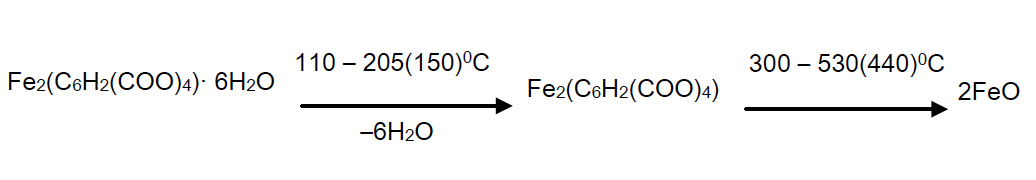
Decomposition of the complex compound of hexaaqua bis benzene 1, 2, 4, 5 tetracarbonate of diiron (II) begins at 110°С in the temperature range of 110 – 205°С and is accompanied by a shallow, but a clear endothermic with the peak at 150°С and corresponds to the removal of six molecules of water.
As it is seen from the thermograms of the complex compound (Figure 3), all six water molecules leave the crystal lattice at one stage and are focal points, since their removal temperature is too high.
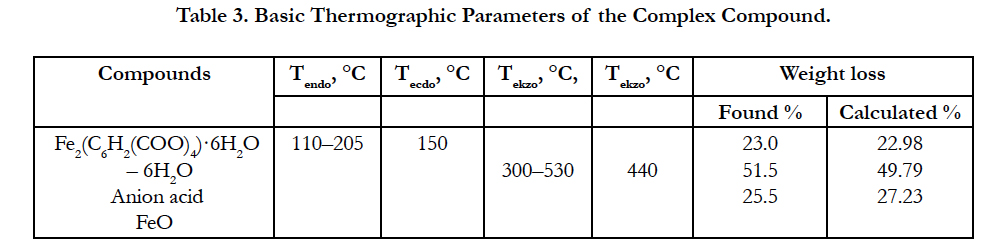
Experimental mass loss value is 24% (calculated 22.98%).
Removal of the water molecules with one precise endothermic effect and a sufficiently high temperature (110 – 205°С) shows that they are coordinated and quite strongly linked to the central atom. Its zinc analogue has 4 moles of water which are removed step by step (2mol, 1.5 mol and 0.5 mol) [13].
Intermediate anhydrous complex is stable up to 300°С which is extremely rare for the complex compounds. At 300°С gradually at 300 – 530°С temperature range decomposition of anhydrous complex and parching of an organic residue with a single precise endothermic effect with a maximum 440°С takes place.
Here the weight loss is experimentally 53% (calculated 53,19%). Since on TG curve after the full decomposition of the complex compound, a slight increase in weight and a good agreementbetween the experimental and theoretical values is observed, the oxidation of ion (Fe2+) is due to atmospheric oxygen. As the final product FeO is left. The final black colored product was FeO (found mass 25.5%, calculated mass 27.23%).
Below, a diagram of a solid phase transformation of the complex compound is given:
Basic thermographic data of the complex compound are shown in Table 3.
Thus, the results of thermogravimetric studies showed that the investigated complex has a chemical formula of Fe2(C6H2(COO)4) (H2O)6 which is consistent with the formula obtained by elemental analysis.
IR spectroscopic study also shows the frequencies at 3600-3200 sm-1, in particular at 3416 sm-1 which refer to the asymmetric and symmetric vibrations of OH for the water coordination [14] (Figure 4).
The absorption bands at 1667, 1586 (νa) and 1458, 1376 sm-1 (νs) refer to the carboxyl groups of pyromellitic acid anion [15].
Significance of difference of the values Δ(νa- νs) is respectively 209 and 210 cm-1 and it is in good agreement with the values of monodentate complexes and show that all four carboxyl groups bound to the central atom as in the complex [11].
Thus, the central atom is five coordinated. The coordination of the iron (II) includes two oxygen atoms of two carboxylic groups of two different anions and three oxygen atoms of three water molecules.
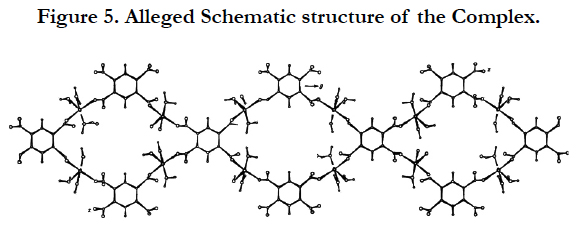
Estimated schematic structure of the complex compound along the axis [11] is shown in Figure 5. In all likelihood coordinately related the water molecules form intermolecular hydrogen bonds both with oxygen of the carboxyl groups, and among themselves.
Conclusions
In the crystal, the layers are interconnected with one another also by the hydrogen bonds, since the unit cell parameter (a) which is perpendicular to the layers; slightly (0.42Å) differs from the parameter(s) of the known complex [11]. Here molecules of coordination water in the different layers play an active role in this interaction.
As the unit cell parameter (a) slightly (0.07Å) differs from the parameter (c) of the known complex, we assume that the layers are slightly displaced relative to each other.
Acknowledgements
Thus, a two-dimensional structure of a complex compound by means of the hydrogen bonds is transformed into 3D structure.
This work was supported by the Science Development Fund under the President of Azerbaijan Republic - Grant № EİF/ GAM-3-2014-6 (21) -24/09/4.
References
- Moci W, Inoue F, Yoshida K, Nakayama H, Kishita M, et al., Synthesis of New Adsorbent Copper(II) Terephthalate. Chem Lett. 12(1): 1219-20.
- Grossman RT, Mc Kane FW Jr (1988) Einsatz von Terephthalaten in Kautschukmischungen. Gummi, Fasern Kunststoffe. 7(41): 356.
- Grossman RT, Tanno D.M.U.S.Pat.5. 083. 235 (1992).
- Grossman RT, Tanno D.M.U.S.Pat.5. 162. 557 (1992).
- Grossman RT, Tanno D.M.U.S.Pat.5. 026.888 (1991).
- Usubaliyev BT, Ganbarov DM, Tomuyeva A Sh (2012) Chemistry and Chemical technology. 2(55): 104-110.
- Ganbarov DM, Tomuyeva A Sh, Usubaliyev BT (2009) Chemistry and Chemical technology. 3(52): 3-11.
- Usubaliyev BT, Tomuyeva A Sh, Munshiyeva MK (2013) Clathrate formation of bisftalat of digidroxomonoakvadik of cadmium and geksagidroksotetra of nickel (II). J gen chem. 7(83): 1181.
- Usubaliyev BT, Ganbarov DM, Tomuyeva A Sh (2013) Formation of compounds including complexes of cadmium, nickel, and cobalt (II) with tetraphthalic acid with a layered structure. Newsletter of Azerb Engineer Academy. 2(5): 100-114.
- Usubaliyev BT, Shabanov AL, Tomuyeva A Sh (2014) Preparation of nonvalence compounds of zinc(II) with 1,2- and 1,4-benzenediacarboxylic acids via self assembly. Russian J gene chem. 7(84): 1183.
- Usubaliyev BT, Shnulin AN, Mamedov Kh S (1982) Crystal and Molecular structure of decahydrate complex of copper with 1,2,4,5-benzenetetracarboxylic acid Coordination chemistry. Coordination chemi. 11(8): 1532.
- Usubaliyev BT, Munshiyeva MK, Aliyeva FB (2016) at all, Synthesis, physical – and structure-chemical research of coordinating compounds of diaquo- 1,2,4,5-benzoltetracarbonat dicopper (II), Bull. Env Pharmacol Life Sci. 5(3): 12-17.
- Usubaliyev BT, Nurullayev VH, Aliyeva FB, Munishiveya Mk, Safarova, et al., Synthesis and structure-chemical research of coordination compounds of tetraaqua bisbenzol-1,2,4,5-tetracarboksilat dizinc (II), Bull Env Pharmacol Life Sci. 5(4): 10-16.
- Bellami (1971) New data on IR spectra of complex molecules. Mir, Moscow.
- Nakaoto K (1991) IR spectra and KR spectra of non-organic and coordinating compounds. Mir, Moscov.