Coagulopathy Caused by the Main Anticoagulant Fractions of Echis carinatus Snake Venom on Blood
Salmanizadeh H1*, Zolfagharian H2, Babaie M1
1 Young Researches and Elites club, Science and Research Branch, Islamic Azad University, Tehran, Iran.
2 Department of Venomous Animals and Antivenom Production, Razi Vaccine and Serum Research Institute, Karaj, Iran.
*Corresponding Author
Hossein Salmanizadeh,
Department of Venomous Animals and Antivenom Production,
Razi Vaccine and Serum Research Institute,
Karaj, Iran.
Tel: +98 9132123192
E-mail: Salmanizadeh64@yahoo.com
Article Type: Research Article
Received: July 10, 2015; Accepted: August 12, 2015; Published: August 14, 2015
Citation: Salmanizadeh H, Zolfagharian H, Babaie M (2015) Coagulopathy Caused by the Main Anticoagulant Fractions of Echis Carinatus Snake Venom on Blood. Int J Nano Stud Technol. 4(4), 93-99. doi: dx.doi.org/10.19070/2167-8685-1500018
Copyright: Salmanizadeh H© 2015 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: The venom of Viperidae snakes is a compound liquid rich in medicinally active proteins and peptides. It is an invasive weapon for preys immobilization, killing and digestion.
Materials and Methods: With a combination of gel and ion exchange chromatography ten sub-fractions were isolated from the E.carinatus venom. Three sub-fractions as anticoagulant sub-fractions were then intravenously injected to mice. Blood sampling was taken before and after injecting these three sub-fractions. The PT, PTT and FT were recorded.
Results: Comparison of the PT before and after injecting three sub-fractions, showed that the blood coagulation time after injection is more than the blood normal coagulation time and also more than the coagulation time after the crude venom injection. This coagulation time difference shows the intense coagulation activity of these sub-fractions which thus significantly decrease the rate of coagulation cascade activity and lead to slow blood coagulation.
Conclusion: Comparison of the PT and PTT after injecting three sub-fractions with this test normal time respectively showed that the rate of the mice blood coagulation extrinsic and intrinsic system activity rate considerably decreases. By comparing the FT after injecting with this test normal time, coagulation cascade intense inactivation and the nonproduction of fibrin can be inferred.
2.Introduction
3.Materials and Methods
3.1. Venom and animals
3.2. Prothrombin Time (PT) test
3.3. Partial Thromboplastin Time (APTT) test
3.4. Fibrinogen Time (FT) test
3.5. Isolation and purification of anticoagulants factors
4.Results
4.1. IEc crude venom gel chromatography
4.2. Study of the anticoagulant activity of the fractions from gel chromatography
4.3. Isolation of subfractions F2 and F3 using ion exchange chromatography
4.4. Study of the F2 and F3 subfractions anticoagulation activity
4.5. Injection of sub-fractions F2B, F3B and F3C
5.Discussion and Conclusion
6.Acknowledgement
7.References
Key Words
Snake Venom; Chromatography; Anticoagulants; Echis carinatus; Blood Coagulation.
Introduction
Of approximately 3000 snake species worldwide, about 600 are venomous. These are found in four snake families: Colubridae, Elapidae, Viperidae and Atractaspididae. Annually, there are more than 2.5 million cases of snake bite, of which about 100,000 are fatal, mostly in rural tropical areas [1].
Disturbances of haemostasis, the system of all reactions that contribute to the effective arrest of bleeding, are among the most severe effects following snake bite and are caused by members of several genera from all four families. The venom producing apparatus of snakes is composed of modified exocrine glands which produce the toxic venom. Venoms are complex mixtures of proteins and peptides possessing a variety of biological activities. Proteins and peptides comprise about 90-95% of the dry weight of the venom. Other components in the venom are metallic cations, carbohydrates, nucleosides, biogenic amines and very low levels of free amino acids and lipids [2].
Snake venoms are complex mixtures containing many different biologically active proteins and peptides. A number of these proteins interact with components of the human hemostatic system. Snake venoms contain many diverse enzymatic activities including, but not limited to, phospholipase, phosphodiesterase, phosphomonoesterase, L-amino acid oxidase, acetylcholinesterase, proteolytic enzymes of the serine proteinase and metalloproteinase classes, arginine esterase, 5'-nucleotidase, hyaluronidase and NAD nucleosidase [3-5].
Anticoagulant activity has been reported in different snake venoms and the responsible proteins have been purified in a number of cases. Anticoagulant action of snake venom proteins is attributed to: (i) the activation of protein C, (ii) the inhibition of blood coagulation factors IX and X by a venom protein that binds to either or both clotting proteins, (iii) a thrombin inhibitor and (iv) phospholipases that degrades phospholipids involved in the formation of complexes critical to the activation of the coagulation pathway [6].
The venom of Viperidae snakes including the Iranian E.carinatus is rich in proteins and peptides effective on the hemostatic system. It selectively influences blood coagulation different factors. The IEc venom is a golden source of bioactive molecules capable of connection to a vast range of objectives including blood coagulation cascade molecules. Also, this venom enjoys many molecules with biological functions applied for hunting [7].
In This study, under in vivo conditions, this venom also displays anticoagulation properties and causes delay in the blood coagulation cascade. This non-coagulability occurs due to the defect and shortage of fibrinogen and of other factors.
Materials and Methods
Sephadex G-75 and DEAE-Sepharose were purchased from Pharmacia (Sweden). CaCl2, Thromboplastin-D and APTT-XL kits were purchased from Fisher Diagnostics (Germany). The other reagents and chemicals were of analytical grade from Fluka and Merck.
60 mg of the Iranian Echis carinatus venom were prepared from the venomous animals unit of Razi Vaccine and Serum Production Research Institute, Hesarak, Karaj, Iran. Eighteen mice of the NIH breed were supplied from the lab animals breeding unit of Razi Vaccine & Serum Production Research Institute, Hesarak, Karaj, Iran. These mice blood samples were centrifuged for 10 minutes at 3000 RPM. The plasma thus obtained was used for the Prothrombin Time, Partial Thromboplastin Time and Fibrinogen Time testes.
The Thromboplastin-D vial is brought to the lab and left there for a while so that its temperature reaches that of the lab. 200 μl of the solution is poured into the lab hemolysis tube using a sampler, and put in 37°C water for 3 minutes. We proceed to pour 100μl of mice plasma into the hemolysis pipe containing 200μl of Thromboplastin-D solution and simultaneously switch the chronometer on. A glass pipe containing 200μl of Thromboplastin- D solution and 100μl of the plasma in question are shaken in a 37°C for 5 seconds. In the lab lamp light, the process of plasma clotting is observed and the time is recorded. By recording the time and using table attached to the kit, INR and the rate of coagulation cascade activity are specified [8, 9].
The APTT-XL solution vial is brought to the lab and left there for a while so that its temperature reaches that of the lab. 100μl of this solution is then poured into the lab hemolysis pipe using a sampler, 100μl of mice plasma are added to it which is left for 3 minutes in 37°C water. We proceed to add 100μl of CaCl2 and simultaneously switch the chronometer on. The preparation is shaken for about 20 second in 37°C water. In the lab lamp light, the process of plasma clotting is observed and the time is recorded [9, 10].
The reagents are taken out of the refrigerator so that their temperature reaches that of the lab (30 minutes before doing the test). 1- Dilution: 0.1 ml of the plasma under test is diluted with 0.9 ml of the test kit diluting buffer to gain the plasma dilution 1:10. 2- Incubation: 0.2 ml of the diluted plasma is poured into the lab hemolysis pipe for incubation for 2 minutes a 37°C. 3- Clot formation: the thrombin containing reagent should have the lab temperature (25°C) throughout the test time (it should never be incubated at 37°C). 2 minutes after incubation, 0.1 ml of the thrombin containing reagent is added to the diluted plasma and the chronometer will be simultaneously switched on. As soon as the first signs of clotting are observed, the time is recorded and the fibrinogen level is determined [11, 12].
Isolation and purification of anticoagulant factors were performed from 50 mg of the E. carinatus crude venom using a combination of gel chromatography and ion exchange chromatography.
Gel chromatography: The IEc crude venom was primarily isolated using the gel chromatography (Sephadex G-75) column which initially gained equilibrium using 20 mM ammonium acetate buffer 20 mM (pH 6.8). That is, the column input and output pH became the same. 50 mg of the IEc crude venom were dissolved lyophilized in 4 ml of ammonium acetate buffer. The solution was then centrifuged for 15 min at 4°C at 14000 rpm. The supernatant was isolated and gradually injected into the gel chromatography (Sephadex G-75) column using a special syringe. The sample was then well absorbed by the column which was then automatically eluted with ammonium acetate buffer using an automatic collector at the flow rate of 60ml/h for 24 h. The absorption of the resulting pipes was read using a spectrophotometer at 280 nm and relevant absorption curve was drawn in terms of the pipes number. For taking the poison ammonium acetate buffer out of the solutions, each of the peaks was dialyzed for 24 h with distilled water. After dialysis, the fractions were concentrated at 4 °C with sucrose.
Ion exchange chromatography: The ion exchange chromatography column gained equilibrium with buffer Tris-Hcl 0.05 mM, (PH 8.2) that is the input buffer was the same as the output buffer. Form the peaks obtained from gel chromatography; the fraction which enjoyed coagulation activity was exposed to ion exchange chromatography for more isolation and subfractionation. Initially, a certain amount the chromatography peaks (anticoagulant fractions) (5cc) was gradually entered in the column (4°C) which was then eluted with buffer Tris-Hcl 0.05 mM. In continuation, the column was eluted with buffer Tris-Hcl, 0.05 mM, gradient buffer; pH 8.2 (linear gradient of NaCl concentration from 0.0 to 0.5 mM). The ion exchange chromatography output solution was collected by an automatic collector at flow rate 20 ml/h for 24 h. The collected pipes absorption was read using a spectrophotometer at 280 nm and relevant optical absorption curve was drown in terms of the pipes number. Dialysis and taking the buffer out of the peaks and concentration were performed just like in gel chromatography [13-15].
Results
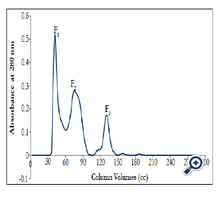
By performing gel chromatography, three peaks or fractions were obtained (Figure 1), which respectively labeled F1, F2 and F3. The F1, was considered as the peak with the highest amount of protein. According to the gel chromatography isolation process based on molecular weight, peaks or fractions respectively containing less total protein will exit from the gel chromatography column. Peaks F2-F3 thus contains proteins with molecular weights lower than that of F1, respectively.
According to Table1, by conducting the PT test on mice plasma, it was known that fraction F2, F3, enjoys a higher antiacoagulation time relative to F1 fraction.
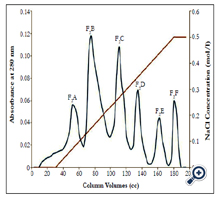
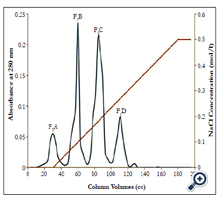
Fraction F1 was discarded, because contain coagulant proteins. Two peaks of F2 and F3, which are major anticoagulation fractions, were ion exchange chromatographed. These two peaks subfractions were thus obtained according to the following figures ( Figure 2 and 3). According to Figure 2, fraction F2 was isolated into six subfractions and according to Figure 3, F3, into four subfractions.
The PT test was frequently conducted on human plasma using subfractions F2 and F3. These results (p ≤ 0.05) showed a significantly anticoagulation activity of sub-fractions F2B, F3B and F3C when compared with others. The mean PT obtained for subfractions F2B was 34.5s, for subfractions F3B more than 300 s and for subfractions F3C, 69s, compared with the normal time, this interval is lower, showing the intense coagulation properties of these subfractions. For more investigation into the coagulation activity, these subfractions were selected for injection into mice.
Each of sub-fractions F2B, F3B and F3C were intravenously (i.v.) injected to three mice of the NIH breed. Tables 2, 3, and 4 and show the results of the PT, PTT and FT tests before and after injection.
Discussion and Conclusion
Snake venom is a golden source of bioactive molecules capable of connection to a vast range of molecular objectives including blood coagulation cascade molecules. The IEc venom is one of the coagulation venoms whose performance is like that of Thromboplastin. Under in vivo conditions, this venom also displays anticoagulation properties and causes delay in the blood coagulation cascade. This noncoagulability occurs due to the defect and shortage of fibrinogen and of factors 5 and 8.
Fractions F1, F2 and F3 were obtained by gel chromatography. By performing the Prothrombin Time test on mice plasma, the blood coagulation time showed an incremental cascade using any of these fractions so that fraction F1 showed the least coagulation time and fraction F3 displayed the highest coagulation time (Table 1). Fractions F2 and F3 were thus considered as anticoagulation fractions. Two peaks F2 and F3, which are major anticoagulation fractions, were ion exchange chromatographed. Fraction F2 generated six subfractions F2A to F2F and fraction F3 made four subfractions F3A to F3D. Each of (anticoagulants) subfractions F2B, F3B and F3C was intravenously injected to three NIH mice.
The mean PT was equal to 13.56s before the injection of subfraction F2B to the mice. This mean value was equal to 61.6s after the injection of this sub-fraction to the mice (Table 2). This considerable and significant increase in the PT test shows the intrinsic anticoagulation property of this sub-fraction as an anticoagulation factor. According to Table 2, if the PT test time becomes 38.2s, the coagulation cascade activity rate will become %18.6 with INR of 5.2. The mean PT which equals 61.6s after the injection of sub-fraction F2B has therefore even become less than 38.2s and the blood coagulation cascade extrinsic pathway activity rate has dropped below %18.6. It can thus be reasoned that the coagulation system has been nearly disturbed in the presence of this sub-fraction. The normal mean PTT was 36.4s while became 47.3s after the injection of sub-fraction F2B. The mean different is not too much. It however shows the blood coagulation intrinsic pathway less activity; this (blood coagulation intrinsic pathway) activity decrease displays the sub-fraction F2B anticoagulation effect. The normal mean FT of sub-fraction F2B was equal to 22.5s. This mean value for the FT after the injection of this sub-fraction became 96s. This time difference shows fibrinogen intense destruction and nearly nonproduction of fibrin. It of course indirectly displays the intense shortage of the coagulation factors exiting in the pathway from blood coagulation to fibrin production.
The mean PT was 13.13s before the injection of sub-fraction F3B. This mean value was unspecified after the injection of this subfraction to the mice, for the coagulation time was more than five minutes (Table 3). This time is by no means significant and shows the blood coagulation cascade inactivity and extrinsic pathway. This considerable and significant increase in the PT test displays the intense anticoagulation property of this sub-fraction as an anticoagulation factor. This sub-fraction is thus a very strong anticoagulant which has completely disturbed the system.
The mean PT was equal to 12.6s before the injection of subfraction F3C. This mean value was equal to 121.3s after the injection of this sub-fraction to the mice (Table 4). This considerable and significant increase in the PT test shows the intense anticoagulation property of this sub-fraction as an anticoagulation factor. Regarding Table 4, if the PT test time becomes 38.2s, the coagulation cascade activity rate will become %18.6 with INR of 5.2. The mean PT which equals 121.3s after the injection of sub-fraction F3C has thus even become less than 38.2s and the blood coagulation cascade extrinsic pathway activity rate has dropped below %18.6. It can thus be reasoned that the coagulation system has been nearly disturbed in the presence of this sub-fraction which is an anticoagulant stronger than F2B. The normal mean PTT before injection was 39.4s while it became 71.6 s after the injection of sub-fraction F3C. This mean difference shows much less activity of the blood coagulation intrinsic pathway. This activity decrease displays the intense anticoagulation effect of sub-fraction F3C. The mean FT before the injection of sub-fraction F3C equaled 18.8s. This mean value for the FT after the injection of this sub-fraction became 110 s. This time difference shows fibrinogen intense destruction and fibrin nonproduction. It of course indirectly displays the intense shortage and evacuation of the coagulation factors exiting in the cascade from blood coagulation to fibrin production.
As a result, sub-fraction F3B is an anticoagulant stronger than the previous two subfractions. The mean PTT before injection or the normal mean PTT was 37.2s while this mean value became 81s after the injection of sub-fraction F3B. This mean difference shows the blood coagulation intrinsic pathway very much less activity. This activity decrease displays the sub- fraction F3B anticoagulation effect. The normal mean FT or the mean FT before the injection of sub-fraction F3B was 20.1s. This mean value for the FT after the injection of this sub- fraction was unspecified. This time difference shows the intense destruction of fibrinogen and fibrin nonproduction. It of course indirectly displays the intense shortage of the coagulation factors exiting in the cascade from blood coagulation to fibrin production.
Banerjee et al. purified and identified two anticoagulation proteins, hemextin A and hemextin B which were included in three-finger proteins and which had a synergic action, from the venom of African Ringhals cobra (Hemachatus haemachatus). Hemextin A showed a medium anticoagulation activity. Hemextin B did not however show such an activity. Anyway, hemextin B reacts with hemextin A to make hemextin AB complex which synergically promotes the coagulation activity. PT assessment in this study showed that these proteins have been formed from complex 1:1. By more effective study, it was known that hemextin A and hemextin AB complex increase the coagulation time by disturbing the extrinsic pathway tenas complex activity. More studies showed that hemextin AB complex enjoys the potential to inhibit the proteolytic activity of FVIIa and its complexes [16].
The venom of Vipera russellii was isolated into 13 fractions using the DEAE-Sephadex A-50 chromatography column. Fraction III enjoyed the anticoagulation power and phospholipase A2 activities and fraction XI had procoagulation and caseinolytic activities. Both fractions were further purified by gel chromatography on the S-200 column. It was a base protein which was a phospholipase A with the enzymatic activity [17].
Lectin-like proteins of the C type have a vast range of biological activities including the anticoagulation and platelet regulating ones. They have yet no lectin activity. These enzymes consist of heterodimers or heterodimers oligomers while the C type lectins extracted from snake venom are uniquely homodimer or homooligidimer. In the recent decade, some lectin- like proteins of the C type including the blood coagulation IX/X factor linking protein and Botrocetin were isolated, sequencialized and identified from diversified venoms. Besides, Rvv-X (factor X activator) and carinactivas 1 (Prothrombin activator) are metalloproteinases consisting of two lectin-like domains of the C type which are domain Gla of factor X and Prothrombin, respectively [18].
Snakes venoms have many molecules with biological functions used for hunting. Any compound of snakes' venoms has a specific objective in hunting and changes its own objective biological function. Some of these molecules were identified, recognized and cloned for the first time, for they can have clinical applications. The Activated Coagulation Time (ACT) and the clot formation rate have been used to screen and study the coagulation properties of 28 venoms of snakes. The venom of Dabia russellii, Bothrops asper, Bothrops moojeni and Crotalus oreganus helleri have coagulation activities. Other venoms like the venom of Crotalus atrox and two venoms of Naja pallida have anticoagulation activities. A significant increase in the ACT and a significant decrease in the clot formation rate were observed and studied after adding these venoms. Venoms of the same species did not always have similar ACT and clot formation rate. These properties are however a perfect way to determine snakes venoms coagulation and anticoagulation activities [19].
Phospholipase A2, an anticoagulation factor was isolated from Naja kaouthia (Indian cobra) venom by a combination of ion exchange chromatography and gel chromatography. This purified protein was called NK-PIA2-I. This protein showed a highly intense anticoagulation activity and has indirectly destructive activities on the liver and heart tissues. It however showed less toxic activity. It indirectly performs hemodialysis and forms edema. It displayed a destructive activity on the lungs tissue in comparison with all the venom [20].
A metalloproteinase, NN-PF3, enjoying nontoxic activities and anticoagulation properties, has been isolated from Naja naja naja (Indian cobra) venom by a combination of column chromatography and electrophoresis. NN-PF3 was injected in a nonlethal dose of 15mg/kg of the mice body weight. It is free of hemorrhagic, myotoxic and cytotoxic activities. It is a protein with weak edema activities. It is however very strongly anticoagulatively active and its effect with its dose or concentration and its relevant time were observed [21].
Naja naja naja (Taiwanese cobra) venom was isolated to 19 fractions by CM-Sephadex C-50 column chromatography. Fractions V and VII had anticoagulation activities, fractioned by gel chromatography on Sephadex G-50 and of which a phospholipase A2 compound activity and inhibition effect on platelet aggregation stimulated by it were identified. It had these two activities. Its anticoagulation activity can be inhibited by phospholipid or platelet factor III. XVII was also an anticoagulation fraction further refractionated by gel chromatography. Its purified compound showed that it is a cardiotoxin with a weak anticoagulation activity, causing hemolysis. It had the potential to stimulate platelet accumulation. Thromboelastographic studies showed that the cobra venom anticoagulation action is due to the venom common phospholipase A2 and cardiotoxic effects [22].
Proteins with anticoagulation and antiplatelet activities were purified from the venom of Austrelaps superbus (copperhead) by gel, ion exchange and HPLC chromatography. These purified proteins were named Superhins I and II. Both proteins displayed phospholipase A2 activities and expressed weak anticoagulation effects, while the PT was tested in an assessment phase. The study of the properties of these two enzymes revealed that they inhibit the blood coagulation cascade extrinsic pathway performance. Results showed that the activities of these two enzymes prevent the formation and activity of the blood coagulation cascade extrinsic pathway tenase. They do not however prevent the prothrombinase complex activity, and they act like other weak anticoagulation phospholipases [23].
The pseudothrombin of Agkistrodon acutus leads to a specific increase in the blood coagulation general time and one phase in the plasma PT. It also causes a specific decrease at the fibrinogen level. Delay in blood coagulation formation by the pseudothrombin enzyme was mainly due to the plasma fibrinogen level decrease. This pseudothrombin enzyme made no significant change in the blood pressure heart rate and respiration rate in a defibrinating dose or concentration (0.1 mg/kg intravenous injection). It did not stimulate platelet accumulation either. Using this pseudothrombin enzyme, no fibrin transverse linkage was formed. The pseudothrombin enzyme can digest the fibrin α chain [24].
The venoms of Cerastes cerastes and Cerastes vipera snakes were anticoagulant at high concentrations and coagulant at low concentrations. Each of the venoms was fractionated into eight fractions by G-100 Sephadex gel chromatography. In two species, there were two anticoagulation fractions. Anticoagulation activities in two fractions mainly directly or indirectly lead to fibrinogenolysis. The coagulation activity is perhaps due to activation in multiple loci (places) in the blood coagulation cascade [25].
Chopra and Chowhan showed that the Echis carinatus venom in vivo injection leads to fibrinolysis leading to the coagulation time specifically observable increase. Taylor et al. observed that this venom is a very strongly active coagulation factor the injection of which will produce cardiovascular fibrin clots within some minutes due to blood noncoagulability. In 1962, Kornalik reported that this venom has a pseudothromboplastin action and can activate the fibrinolysin enzyme [26].
The venom of Echis carinatus including the Iranian Echis carinatus in one of the coagulation venoms whose function is a pseudothromboplastin action. Under in vivo conditions however, his venom will also generate high noncoagulation which is due to fibrinogen deficit in factors 5 & 8.
Acknowledgement
Authors would like to thank all members of the Department of Venomous Animals and Antivenom Production. Authors would also like to thank Mr. Askari and Jafari in enzyme section and Ms. Khame chiyan in Department of serum production for their great help.
References
- White J (2005) Snake venoms and coagulopathy. Toxicon 45(8): 951-967.
- Pradeep KM, Basheer MP (2011) Snake bite: Biochemical changes in blood after envenomation by viper and cobra. J Med Allied Sci 1(1): 36-41.
- Kini RM (2006) Anticoagulant proteins from snake venoms: structure, function and mechanism. Biochem J 397(3): 377-387.
- Sajevic T, Leonardi A, Krizaj I (2011) Haemostatically active proteins in snake venoms. Toxicon 57(5): 627-645.
- Loubna H, Gargioli C, Castelli S, Rufini S, Djebari LF (2010) Purification and characterization of a fibrinogenolytic and hemorrhagic metalloproteinase isolated from Vipera lebetina venom. Biochimie 92(7): 797-805.
- Markland FS (1998) Snake venoms and the hemostatic system. Toxicon 36(12): 1749-1800.
- Suttie JW, Jackson CM (1977) Prothrombin structure, activation and biosynthesis. Physiol Rev 57(1): 1-70.
- Ghorbanpur M, Zare Mirakabadi A, Zokaee F, Zolfagharian H, Rabiei H (2009) Purification and partial characterization of a coagulant serine protease from the venom of the Iranian snake Agkistrodon halys. J Venom Anim Toxins incl Trop Dis 15(3): 411-423.
- Rizzo F, Papasouliotis K, Crawford E, Dodkin S, Cue S (2008) Measurement of prothrombin time (PT) and activated partial thromboplastin time (APTT) on canine citrated plasma samples following different storage conditions. Res Vet Sci 85(1): 166-170.
- García-Manzano A, González-Llaven J, Lemini C, Rubio-Póo C (2001) Standardization of Rat Blood Clotting Tests with Reagents Used for Humans. Proc West Pharmacol Soc 44: 153-155.
- Mackie IJ, Kitchen S, Machin SJ, Lowe GD (2003) Guidelines on fibrinogen assays. Br J Haematol 121(3): 396-404.
- Jennings I, Kitchen DP, Woods TA, Kitchen S, Walker ID (2009) Differences between multifibrin U and conventional Clauss fibrinogen assays: data from the UK National External Quality Assessment scheme surveys. Blood Coagul Fibrinolysis 20(5): 388-390.
- Ghorbanpur M, Zare Mirakabadi A, Zokaee F, Zolfagharian H (2010) Identification and partial purification of an anticoagulant factor from the venom of the Iranian snake Agkistrodon halys. J Venom Anim Toxins incl Trop Dis 16(1): 96-106.
- Masci PP, Whitaker AN, de Jersey J (1988) Purification and characterization of a prothrombin activator from the venom of the Australian brown snake, Pseudonaja textilis textilis. Biochem Int 17(5): 825-835.
- Oyama E, Takahashi H (2003) Purification and characterization of a thrombin like enzyme, elegaxobin II, with lys-bradykinin releasing activity from the venom of Trimeresurus elegans (Sakishima-Habu). Toxicon 41(5): 559- 568.
- Banerjee Y, Mizuguchi J, Iwanaga S, Kini RM (2005) Hemextin AB Complex - A Snake Venom Anticoagulant Protein Complex That Inhibits Factor VIIa Activity. Pathophysiol Haemost Thromb 34(4-5): 184-187.
- Teng CM, Chen YH, Ouyang C (1984) Purification and properties of the main coagulant and anticoagulant principles of Vipera Russell II snake venom. Biochim Biophys Acta 786(3): 204-212.
- Morita T (2005) Structures and functions of snake venom CLPs (C -type lectin-like proteins) with anticoagulant, procoagulant, and platelet modulating activ ities. Toxicon 45(8): 1099-1114.
- Suntravat M, Nuchprayoon I, Pérez JC (2010) Comparative study of anticoagulant and procoagulant properties of 28 snake venoms from families Elapidae, Viperidae, and purified Russell’s viper venom-factor X activator (RVV-X). Toxicon 56(4): 544-553.
- Doley R, Mukherjee AK (2003) Purification and characterization of an anticoagulant phospholipase A(2) from Indian monocled cobra (Naja kaouthia) venom. Toxicon 41(1): 81-91.
- Jagadeesha DK, Shashidhara R, Girish KS, Kemparaju K (2002) A non-toxic anticoagulant metalloprotease: purification and characterization from Indian cobra (Naja naja naja) venom. Toxicon 40(6): 667-675.
- Teng CM, Kuo YP, Lee LG, Ouyang CH (1987) Characterization of the anticoagulants from Taiwan cobra (Naja naja atra) snake venom. Toxicon 25(2): 201-210.
- Subburaju S, Kini RM (1997) Isolation and purification of superbins I and II from Austrelaps superbus (copperhead) snake venom and their anticoagulant and antiplatelet effects. Toxicon 35(8): 1239-1250.
- Ouyang C, Teng CM (1978) in vivo effects of the purified thrombin-like and anticoagulant principles of Agkistrodon acutus (Hundred pace snake) venom. Toxicon 16(6): 583-593.
- Labib RS, Azab MH, Farag NW (1981) Effects of Cerastes cerastes (Egyptian sand viper) and Cerastes vipera (Sahara sand viper) snake venoms on blood coagulation: Separation of coagulant and anticoagulant factors and their correlation with arginine esterase and protease activities. Toxicon 19(1): 85-94.
- Chopra RN, Chowhan JS, De NN (1935) An experimental investigation into the action of the venom of Echis carinatus. Indian J Med Res 23: 391- 405.
- Marsh N, Williams V (2005) Practical applications of snake venom toxins in haemostasis. Toxicon 45(8): 1171-1181.