A Comparative Study on the Textile Effluent Decolourization by Using Synthesized Nano Particle and Procured Nano Particle
Narayanappa Madhusudhana1, Kambalagere Yogendra2, Kittappa M Mahadevan3
1 Dept of Environmental Science, Kuvempu University, Shankaraghatta-577451, Karnataka, India.
2 Assistant Professor, Dept of Environmental Science, Kuvempu University, Shankaraghatta-577451, Karnataka, India.
3 Professor, Dept of Chemistryk, Kuvempu University, Shankaraghatta-577451, Karnataka, India.
*Corresponding Author
Kambalagere Y,
Assistant Professor,
Dept of Environmental Science,
Kuvempu University,Shankaraghatta-577451,
Karnataka, India.
E-mail: yogendraku@gmail.com
Article Type: Research Article
Received: September 25, 2014; Accepted: October 08, 2014; Published: October 25, 2014
Citation: Madhusudhana N, Kambalagere Y, Mahadevan K M (2014) A Comparative Study on the Textile Effluent Decolourization by Using Synthesized Nano Particle and Procured Nano Particle. Int J Nano Stud Technol. 3(5), 59-66. doi: dx.doi.org/10.19070/2167-8685-1400012
Copyright: Kambalagere Y© 2014 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Semiconductor photocatalysis often leads to partial or complete mineralization of organic pollutants. Upon irradiation with UV/visible light, semiconductors catalyze redox reactions in presence of air/O2 and water. Here, the potential of a common semiconductor, ZnO, has been explored as an effective catalyst for the photodegradation of textile effluent. The effects of process parameters like, catalyst loading, initial dye concentration and pH on the extent of photo degradation have been investigated. It is difficult to give a general picture of the kinetics using these wastewaters but the process was found to be quite effective for the decolourization of textile waste water. In this work, a detailed investigation was done on photocatalytic degradation for selected textile effluent. The ZnO nano particle was synthesized by using fuel urea by solution combustion method. This nano particle was characterized by using X-ray diffraction (XRD), Scanning Electron Micrograph (SEM) and UV absorbance spectroscopy. The average size was found to be 41 nm. The band gap of the nano particle was found to be 3.2 eV and was tested for the photocatalytic degradation by varying parameters such as catalyst concentration, pH and dye concentration. Also, the efficiency of the synthesized nano particle was compared with the procured TiO2 of size < 25 nm.
2.Introduction
3.Materials and Methods
3.1 Synthesis of Zno Nano Particle by Solution Combustion Method
3.2 X-Ray Diffraction Technique (XRD)
3.3 X-Ray Diffraction Studies of Zno
3.3 SEM Study of Zno
3.4 UV-Absorbance of Spectroscopy
3.5 UV-absorption study of ZnO
4.Results and Discussion
4.1 Photocatalytic Experimental Procedure: Initial Study of the Textile Effluent
4.2 Photocatalytic experimental procedure: Comparative study of photocatalytic decolourization of coloured textile effluent using the ZnO nano particles over procured TiO2
4.3 Effect of Photocatalysts on Photocatalytic Decolourization of Textile Effluent
4.4 Photocatalytic Experimental Procedure: Study on Photocatalytic Decolourization of Textile Effluent Using ZnO Nano particle
4.5 Effect of Catalyst Concentration on Textile Effluent
4.6 Mechanism of Photocatalytic Decolourization
4.7 Effect of pH on Textile Effluent
4.8 Effect on Different Concentration of Textile Effluent
4.9 Effect of Sunlight Irradiation on Textile Effluent
5.Conclusion
6.References
Key Words
Degradation, Nano particles, Photocatalyst, Textile effluent, TiO2, ZnO.
Introduction
The impacts on the environment by textile industry have been recognized for some time, both in terms of the discharge of pollutants and of the consumption of water and energy [1]. Environmental problems of the textile industry are mainly caused by discharges of wastewater. The textile sector has a high water demand. Its biggest impact on the environment is related to primary water consumption (80–100m3/ton of finished textile) and wastewater discharge (115–175kg of COD/ton of finished textile, a large range of organic chemicals, low biodegradability, colour and salinity). Many researchers have considered that the removal of color from wastewaters is often more important than the removal of other organic colorless chemicals. Decolorization of effluent from textile dyeing and finishing industry was regarded important because of aesthetic and environmental concerns. Therefore, reuse of the effluents represents an economical and ecological challenge for the overall sector [2]. Textile processing employs a variety of chemicals, depending on the nature of the raw material and product [3]. The effluents resulting from these processes differ greatly in composition, due to differences in processes, used fabrics and machinery [4].
Semiconductors are used to degrade organic pollutants in water to less harmful inorganic material. There have been numerous studies carried out across the globe focusing on the decolorization of textile wastewater [5,6]. The importance of these types of research is being increasingly in the recent and has become a subject of major public health concern and scientific interest. The treated water may be recycled in the same factory or reused in other applications such as other industries or agriculture that require a less quality water. This is considered to be very excellent means for saving huge amounts of water, especially, in the countries which are suffered with water deficiency.
In the present study, an attempt has been made to remove the colour of the textile effluent collected from Himatsingka Linens, Hassan. The collected textile wastewater was a complex mixture of number of dyes and other ingredients used for the dyeing process. The concentrated textile wastewater was diluted with potable water for laboratory studies. TiO2 of Size < 25 nm has been procured from sigma Aldrich and compared with the synthesized ZnO nano particle. The nano particle was characterized by Xray diffraction (XRD) and Scanning Electron Micrograph (SEM) studies. The decolorization of one of the textile effluent solution (3%) was experimented in presence of sunlight irradiation.
Materials and Methods
The TiO2 nano particle of size < 25 nm was procured from sigma-aldrich, Mumbai. ZnO nano particle was synthesized in the laboratory by Zn(NO3)2.6H2O using fuel urea, because it seem to be the convenient fuel which is easily available for the synthesis of the ZnO nano particle. The chemicals like Zinc nitrate Zn(NO3)2.6H2O (99%, AR) and urea (NH2 CO NH2) (99%, AR) were obtained from Hi-media chemicals, Mumbai and used as received. The UV-VIS single beam spectrophotometer 119 (Systronics) has been used for recording absorbance at λmax.
The solution combustion process is an exothermic redox (oxidation and reduction reactions taking place simultaneously) reaction between an oxidizer and a fuel. When the heat is more evolved than the heat required for the reaction, the system becomes selfsustained. Also the exothermicity of such reactions takes the system to a high temperature. Hence, this process, popularly known as self-propagating high temperature synthesis, which is also called fire synthesis. The interesting feature of the process is that the sample once ignited continues to burn to consume itself [7,8].
The stoichiometric amounts of Zinc nitrate, Zn(NO3)2.6H2O (17.85g) was dissolved in a minimum quantity of water along with (NH2 CO NH2) (6g) in a silica crucible (with a volume of 100 cm3). The mixture was introduced into the muffle furnace which was preheated at 600°C. Initially the solution foils, boils and undergoes dehydration followed by decomposition with the evolution of gases (N2 and CO2). Then, it burns to yield the residue. The gases evolved not only yield fine particles of metal oxides but also help to dissipate the heat which inhibits sintering of the product. Thus, combustion reaction was completed within a few minutes [9]. The combustion reaction for the synthesis of ZnO by the redox mixture method can be written as:
6 Zn(NO3)2 + 10 NH2CONH2 → 6 ZnO + 10 CO2 + 20 H2O + 16 N2<
The equivalance ratio (ɸe) in the above case is calculated using Oxidizing Valency (OV) and Reducing Valency (RV) (Table 1) (Fig 1):
OV of Zn(NO3)2,
1Zn = +2, 2N = 0, 6O = –12, Total = +2 –12 = –10.
RV of NH2CONH2,
1C = +4, 4H = +4, 2N =0, 1O = –2, Total = +4+4–2 = +6.
(ɸe , O/F)= 10/6 = 1.66
i.e., for every one mole of Zn(NO3)2 1.66 moles of urea required.
XRD was performed by Rigaku diffractometer using Cu-Kα radiation (1.5406 Å) in a θ-2θ configuration [10,11]. According to the Debye Scherrer’s formula:
Debye Scherrer's formula D=[Kλ ∕ βCOSθ] ------ (Eq.1)
Where D = Thickness of the crystallite
K = 0.90 the Scherrer’s constant (dependent on crystallite shape)
λ = X-ray wavelength
β = the peak width at half-maximum (FWHM)
θ = the Bragg diffraction angle
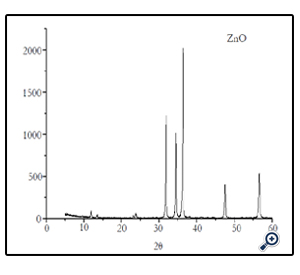
The powdered sample of ZnO nano particle was examined by XRD and analysis was carried out on fresh sample to assess the purity of the expected phases and the degree of crystallization i.e., size, composition and crystal structure. XRD was performed by Rigaku diffractometer using Cu-Kα radiation (1.5406 Å) in a θ-2θ configuration. According to the XRD the average crystallite size of ZnO was found to be 41nm (Fig. 2).
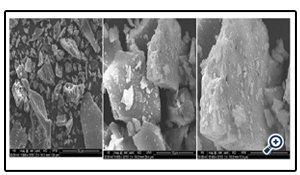
The Figures illustrate SEM photographs of single crystals of ZnO. The photographs revealed plate like crystal morphology which looks like scattered. The individual plates are having sharp edges. The enlarged image shows the particles are attached on the other particles (Fig. 3).
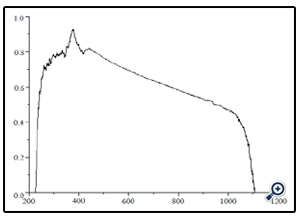
Here Absorption spectra of the ZnO metal oxide nano particle was recorded using UV-VIS spectrophotometer (Ocean Optics DH-2000) over the wavelength range 200-1200nm at Nano Research Laboratory, Department of Nanotechnology, Kuvempu University. From this spectrum, it has been inferred that the nano particles have sufficient transmission in the entire visible and IR region. The band gap energy of the ZnO nano particle was calculated using the following simple conversion equation. The band gap equation is calculated using the Planck’s equation as follows.
e = hC ∕ λ
h= Planck’s constant, C= Velocity of light (speed of light),λ=wavelength of light
3 × 108 m/s, λ = --- × 10-9 nm
Band gap energy (eV) = 4.135 × 10-15 × 3 × 108 × 109
Band gap energy (eV) = [ 1240 ∕ wavelength (nm) ]------(Eq.2)
In the present work powdered sample of different metal oxide nano particles were examined by using UV absorption studies.
The band gap energy of ZnO is found to be 3.2 eV, and with this we can say that the band gap of the semiconductors has been found to be particle size dependent [12]. It is reported that, according to the band theory each solid can be characterized by two energetic bands, a valence band (VB), which possesses lower energy, completely filled with electrons (at least at 0°K), and a conductivity band (CB), with higher energy, empty at 0°K. The energetic distance between them amounts to 0.7-3.5 eV for semiconductors and is called a forbidden band or a band gap, EG. The distance between the valence and conductivity bands determines electronic properties of the solid, e.g. electric conductivity. For comparison, in metals VB overlaps CB, in isolators EG amounts to 6-7 eV. Band gap values also determine the colour of the semiconductor, because they absorb light having energy equal to or higher than EG energy. Such light absorption causes electron transfer from a valence band to a conduction band. The energy of visible light lies in the region of 1.5 (red) - 3.0 eV (violet). Thus, the semiconductors having a narrow band gap of about 1.5 eV are black, those having a band gap of about 3.0 eV white [13].
Raw wastewater sample was collected from homogeneous tank of the textile industry. Initially, sample was analyzed for some primary parameters like pH, Temperature, Odour, Colour, and COD. As the collected textile wastewater was highly concentrated, the sample was diluted with potable water before photocatalytic decolourization. The values of various wastewater parameters before treatment are shown in table 3.
Photocatalytic experiments were carried out in presence of direct sunlight of intensity between 100000 to130000 lux (recorded by using TES 1332A digital Lux meter). The experiments were carried out between 10 am to 1 pm and 3ml of raw effluent was diluted 1000 ml of potable water to make the aqueous solution of 3% concentration. In all photocatalytic experiments, 100ml of 3% textile effluent solution was taken in 100ml Borosil beakers. The UV-VIS spectrophotometer 119 (Systronics) was used for the determination of absorbance in the range of 200 to 800nm. The λmax of textile effluent was found to be 440nm. A known amount of dosage (0.5g/100ml) of the different nano particles; TiO2, ZnO were added to different beakers containing textile effluent solution and the beakers were kept in the sunlight for photocatalytic activity. Further experiments were conducted based on the decolourization results obtained from the photocatalytic activity of the catalysts.
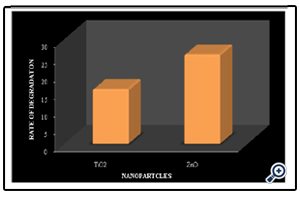
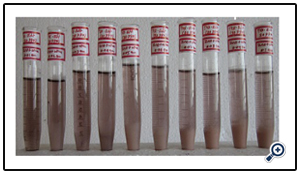
Blank experiments were performed under direct sunlight without the addition of catalysts and no decolourization was observed. A known concentration of TiO2 and ZnO (0.5g/100ml) were added to two beakers containing textile effluent solution and kept in the sunlight for photocatalytic activity. The results showed that the nano particle (ZnO) has exhibited more photocatalytic activity than the procured TiO2. A decolourization of 15.60% was recorded for TiO2 nano particle and 25.55% for ZnO was recorded (Fig. 5) (Photo 1). Photocatalytic decolourization of the azo dye is mainly due to the hydroxyl radical attack on the dye molecule [14,15]. The production of hydroxyl radicals of the TiO2 catalyst may be very less when compared with the synthesized nano particles which have exhibited high degradation in presence of sunlight. Based on the results further studies were done concentrating on the synthesized ZnO nano particle.
Figure 5. Rate of degradation of textile effluent at 120 minutes [textile effluent =3%, pH=7, TiO2=0.5g and ZnO=0.5g]
In the second phase, the synthesized nano particle was continued for the photocatalytic degradation study. Photocatalytic suspensions from 0.1g, 0.2g, 0.3g upto 1g were tested on the 100 ml textile effluent samples. The suspension pH values were adjusted by using NaOH/HCl solutions using pH meter. Before irradiation, photocatalyst suspension was stirred in the dark to ensure the adsorption equilibrium and was kept in sunlight for the photocatalytic decolourization. At an interval of 30 minutes the suspension was sampled and centrifuged (EBA-Hetlich) at 3000 rpm for 5 minutes to remove photocatalyst particles. The residual concentration of the solution samples was monitored by using UV-VIS spectrophotometer 119 (Systronics) at 440nm. The experiments were conducted for different pH range from 2 to 11 in order to study the efficiency of the nano particles in Acidic, Alkaline and Neutral conditions. The data obtained from the photocatalytic degradation experiments were used to calculate the degradation efficiency ‘D’ (Eq. 3).
D = (A0-At / A0) × 100 -----------(Eq. 3)
Where, A0 is the initial absorbance of dye solution At is absorbance at time ‘t’.
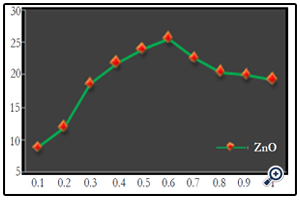
The effect of catalyst concentration on photocatalytic degradation was studied over a range of catalyst amount from 0.1 to 1g/100ml for the textile effluent. The synthesized nano particle has shown appreciable result. The ZnO showed a highest degradation of 25.55% at 0.6g/100ml in 120 minutes (Fig. 6) (Photo 2). The increase in decolourization rate can be explained in terms of availability of active sites on the catalyst surface and sunlight penetration into the suspension as a result of increased screening effect and scattering of light. Further increase in the catalyst amount beyond the optimum dosage for all the nano particles decreases the decolourization by some margin. This may be due to overlapping of adsorption sites as a result of overcrowding owing to collision with ground state catalyst [16]. Since the decolourization was most effective at 0.6g/100ml for ZnO nano particle dosage, the following experiments were continued with same dosages.
Figure 6. Effect of catalyst concentration on textile effluent at 120 minutes [textile effluent=3% at pH=7]
Photo 2. Effect of catalyst concentration on textile effluent at 120 minutes [textile effluent=3% at pH=7]
Nano particle+ hν → (e -CB + h+VB) (Eq. 4)
e-CB + O2 → O2•− (Eq. 5)
H2O + O2•− → OOH• + OH− (Eq. 6)
2OOH• → O2 + H2O2 (Eq. 7)
O2•− + textile effluent → textile effluent -OO•(Eq. 8)
OOH• + H2O + e-CB → H2O2 + OH− (Eq. 9)
H2O2 + e-CB → OH• + OH- (Eq. 10)
H2O2 + O2•− → OH• + OH− + O2 (Eq. 11)
OH•/O2•−/Nano particle•++ textile effluent → textile effluent decolourization (Eq. 12)
The mechanism of photocatalytic activity of the used catalyst nano particle is predicted as follows. Under sunlight irradiation catalyst molecules get excited and transfer electron to the conduction band (Eq. 4). Electron in the conduction band of the catalyst can reduce molecular oxygen and produce the super oxide radical (Eq. 5). Molecular oxygen, adsorbed on the surface of the photocatalyst prevents the hole-electron pair recombination process [17,18]. Recombination of hole-electron pair decreases the rate of photocatalytic degradation. This radical may form hydrogen peroxide or organic peroxide in the presence of oxygen and organic molecule (Eq. 6, 7, 8). Hydrogen peroxide can be generated in another path (Eq. 9). Hydrogen peroxide can form hydroxyl radicals which are powerful oxidizing agents (Eq. 10, 11). The radicals produced are capable of attacking textile effluent molecules and decolourize them (Eq. 12).
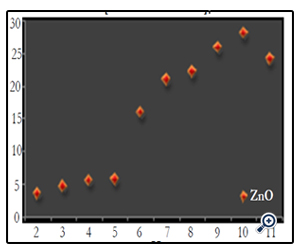
In order to study the effect of pH on the decolourization efficiency of ZnO catalyst, the experiments were conducted at pH ranging from 2 to11. The results showed that, pH significantly affected the decolourization efficiency (Fig. 7) (Photo 3). The decolourization rate of textile effluent for ZnO, the decolourization increased from 3.60% to 27.78% at pH 10 and found to be decreased to 23.84% at pH 11 in a span of 120 minutes for 0.6g/100ml. The maximum decolourization rate for ZnO nano particle was achieved at pH 10. As the collected effluent may be having the anionic dyes which results in more efficient formation of hydroxyl radicals in alkaline medium. Excess of hydroxyl anions increases the formation of OH• radicals. These OH• radicals are the main oxidizing species responsible for photocatalytic decolourization [19]. Above optimum pH, the decrease in decolourization efficiency can be explained on the basis of amphoteric nature of the catalysts. The catalyst surface becomes negatively charged for higher pH values, which causes the electrostatic repulsion between the catalyst and negatively charged dyes [20].
Figure 7. Effect of pH on the photocatalytic degradation of textile effluent at 120 minutes [textile effluent =3%]
Photo 3. Effect of pH on the photocatalytic degradation of textile effluent at 120 minutes [textile effluent =3%]
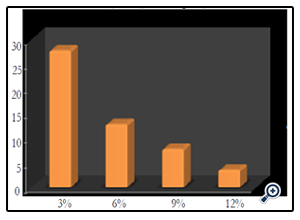
The initial concentration of textile effluent was varied from 3%, 6%, 9% and 12% to study the effect on different concentrations of textile effluent. The decolourization results for ZnO were 27.78% at 3%, 12.81% at 6%, 7.77% at 9% and 3.50% at 12%. (Fig. 8) (Photo 4). These series of experiments illustrated that the decolourization efficiency was inversely affected by the concentration. The decrease in the decolourization with increase in effluent concentration was ascribed to the equilibrium adsorption of dye on the catalyst surface which results in decrease in the active sites This phenomenon results in the lower formation of OH• radicals which were considered as primary oxidizing agents of the organic dye [21]. According to Beer Lambert law, as the initial dye concentration increases, the path length of photons entering the solution decreases. This results in lower photon adsorption on the catalyst particles, and consequently decreases photocatalytic reaction rate [22].
Figure 8. Effect of initial dye concentration on photocatalytic degradation of textile effluent [ZnO/pH = 0.6g/10]
Photo 4. Effect of initial dye concentration on photocatalytic degradation of textile effluent [ZnO/pH = 0.6g/10]
Sunlight irradiation generates the photons required for the electron transfer from the valence band to the conduction band of a semiconductor photocatalyst. The energy of a photon is related to its wavelength and the overall energy input to a photocatalytic process is dependent on the light intensity. Therefore, the effects of both intensity and wavelength are important. In the present study, the effect of the sunlight intensity was studied by keeping the constant wavelength (440nm).
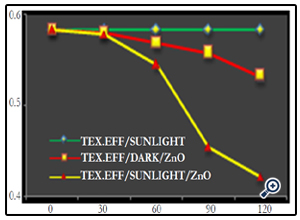
The photocatalytic decolourization of diluted textile effluent (i.e., 3%) conducted under three different experimental conditions were examined, i.e., under sunlight alone, textile effluent/dark/ catalyst and textile effluent/sunlight/catalyst for both the different catalysts. When textile effluent solution was exposed directly to the sunlight, the decolourization was found to be nil during the entire experiments. The decolourization rate was found to increase with increase in irradiation time, for textile effluent/sunlight/ ZnO which achieved 27.78% and for textile effluent/dark/ ZnO 8.74% was recorded with 120 minutes respectively (Fig. 9) (Photo 5).
Figure 9. Effect of sunlight irradiation on photocatalytic degradation of textile effluent in 120 minutes. [ZnO at pH 10]
Photo 5. Effect of sunlight irradiation on photocatalytic degradation of textile effluent in 120 minutes. [ZnO at pH 10]
These results clearly showed that, decolourization occurs more efficiently in presence of sunlight. Under sunlight excitation of catalysts takes place rapidly than in absence of light. The experiment demonstrated that, both sunlight and photocatalyst are needed for the effective destruction of textile effluent, as it has been established that the photocatalytic decolourization of organic matter in the effluent is initiated by the photo excitation of the semiconductor, followed by the formation of electron hole pair on the surface of the catalyst.
Conclusion
In the present work ZnO photocatalyst was prepared by solution combustion method. ZnO was characterized by different technique such as XRD, SEM and UV-vis reflectance. The coloured textile effluent is undesirable for the aquatic environment as it limits the utilization of the water resources. Photocatalytic decolorization is an alternative method to other conventional inefficient physico-chemical and biological methods to treat toxic effluents. The feasibility of photocatalytic decolourization and degradation of the effluent was studied by using the synthesizedmetal oxide nano particle.
The synthesized photocatalyst has shown a maximum decolourization than the procured TiO2. Hence, the obtained results have proved that, photocatalytic decolourization of textile effluent was mainly dependent on the pH of the dye solution and catalyst dosage. The textile effluent achieved maximum colour removal in alkaline medium. The results also revealed that, the sunlight is most efficient source for the photocatalytic activity. Hence the used nano particle can be used to decolorize the coloured effluents.
References
- Kuo WS, Ho PH (2006) Solar photocatalytic decolorization of dyes in solution with TiO2 film. Dyes and Pigments 71: 212-217.
- Li-Rosi O, Casarci M, Mattioli D, De-Florio L (2007) Best available technique for water reuse in textile SMEs (BATTLE LIFE Project). Desalination 206: 614-619.
- Aslam MM, Baig MA, Hassan I, Qazi IA, Malik M, et al(2004) Textile wastewater characterization and reduction of its COD & BOD by oxidation. Electronic Journal of Environmental, Agricultural and Food Chemistry 3: 804-811.
- Bisschops I, Spanjers H (2003) Literature review on textile wastewater characterization. Environmental Technology 24: 1399-1411.
- Pantelis AP, Nikolaos PX, Dionissios M (2006) Treatment of textile dyehouse wastewater by TiO2 photocatalysis. Water Research 40: 1276-1286.
- Chakrabarti S, Dutta BK, (2004) Photocatalytic degradation of model textile dyes in wastewater using ZnO as semiconductor catalyst. Journal of Hazardous Materials 112: 269-278.
- Mimani T, Patil KC (2001) Solution combustion synthesis of nanoscale oxides and their composites. Materials Physics and Mechanics 4: 134-137.
- Yogendra K, Suneel Naik, Mahadevan KM, Madhusudhana N (2011) A comparative study of photocatalytic activities of two different synthesized ZnO composites against Coralene Red F3BS dye in presence of natural solar light. International Journal of Environmental Sciences and Research 1: 11- 15.
- Patil KC, Aruna ST, Mimani T (2002) Combustion synthesis: an update. Current Opinion in Solid State and Materials Science 6: 507-512.
- Girase KD, Patil HM, Sawant DK, Bhavsar DS (2011) Effect of Cu2+ Doping on the Growth and Band Gap Energy of Nano Crystals of Lead Iodate. Archives of Applied Science Research 3: 128-134.
- Habibi MH, Askari E (2011) Photocatalytic degradation of an azo textile dye with manganese doped ZnO nanoparticles coated on glass. Iranian Journal of Catalysis 1: 41-44.
- Sawant DK, Patil HM, Bhavsar DS, Patil JH, Girase KD (2011) Structural and Optical Properties of Calcium Cadmium Tartrate. Archives of Physics Research 2: 67-73.
- Sobczynski A, Dobosz A (2001) Water Purification by Photocatalysis on Semiconductors. Polish Journal of Environmental Studies 10: 195-205.
- Giwa A, Nkeonye PO, Bello KA, Kolawole EG, (2012) Oliveira Campos AMF Solar Photocatalytic Degradation of Reactive Yellow 81 and Reactive Violet 1 in Aqueous Solution Containing Semiconductor Oxides. International Journal of Applied Science and Technology 2: 90-105.
- Madhusudhana N, Yogendra K, Mahadevan KM, Suneel Naik (2011) Photocatalytic Degradation of Coralene Dark Red 2B Azo Dye Using Calcium Zincate Nanoparticle in Presence of Natural Sunlight: An Aid to Environmental Remediation. International Journal of Chemical Engineering and Applications 2: 301-305.
- Subramani AK, Byrappa K, Ananda S, Lokanatha Rai KM, Ranganathaiah C, et al (2007) Photocatalytic degradation of indigo carmine dye using TiO2 impregnated activated carbon, Bulletin of materials science 30: 37-41.
- Di-Paola A, Augugliaro V, Palmisano L, Pantaleo G, SavinovE (2003) Heterogeneous photocatalytic degradation of nitrophenols. Journal of Photochemistry and Photobiology A: Chemistry 155: 207-214.
- Da-Silva CG, Faria JL (2003) Photochemical and photocatalytic degradation of an azo dye in aqueous solution by UV irradiation. Journal of Photochemistry and Photobiology A: Chemistry 155: 133-143.
- Noorjahan, M, Pratap Reddy M, Durga Kumari V, Lavedrine B, Boule P, et al (2003) Photocatalytic degradation of H-acid over a novel TiO2 thin film fixed bed reactor and in aqueous suspensions. Journal of Photochemistry and Photobiology A: Chemistry 156: 179-187.
- Turchi CS, Ollis DF (1990) Photocatalytic Degradation of Organic Water Contaminants: Mechanisms Involving Hydroxyl Radical Attack. Journal of Catalysis 122: 178-192.
- Mirkhani V, Tangestaninejad S, Moghadam M, Habibi MH Rostami Vartooni A (2009) Photocatalytic Degradation of Azo Dyes Catalyzed by Ag Doped TiO2 Photocatalyst. Journal Iranian Chemical Society 6: 578-587.
- Byrappa K, Subramani AK, Ananda S, Lokanatha Rai KM, Dinesh R, et al (2006) Photocatalytic degradation of Rhodamine B dye using hydrothermally synthesized ZnO. Bulletin of Material Sciences 29: 433-438.