Renoprotective and antioxidant renal effects of cilnidipine in DOCA Salt -Treated Albino Rats
Sahar Mohamed Kamal
Department of Pharmacology,Faculty of Medicine, University of Ain-Shams, Egypt.
*Corresponding Author
Sahar Mohamed Kamal,
Department of Pharmacology,
Faculty of Medicine,University of Ain-Shams,
Egypt.
E-mail: saharkamal2003@hotmail.com
Article Type: Research Article
Received: July 16, 2014; Accepted: July 30, 2014; Published: July 31, 2014
Citation: Sahar Mohamed Kamal. (2014). Renoprotective and antioxidant renal effects of cilnidipine in DOCA Salt -Treated Albino Rats, Int J Nano Stud Technol, 03(03), 50-54. doi: dx.doi.org/10.19070/2167-8685-1400010
Copyright: Sahar Mohamed Kamal © 2014 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Aim: Cilnidipine is a dihydropyridine calcium channel blocker (CCB) that blocks both L-type and N- type channels at the smooth muscle in the artery and calcium channels at the presynaptic nerve terminal, respectively. It is dihydropyridine calcium antagonist that possesses a slow-onset, long-lasting vasodilating effect.
The present study is designed to determine the effect of cilnidipine on creatinine clearance, thiobarbituric acid reactive substance, as an index of lipid peroxidation. Additionally, the level of superoxide dismutase enzyme in erythrocyte lysates and kidney catalase enzyme activitiy will be measured. Thirty-six albino rats are divided into 3 separate groups: rats of group 1 are control group, rats of group 2 are administered by deoxycorticosterone acetate (DOCA) salt sc twice/week + 1% NaCl for 2 weeks to be rendered hypertensive) and group 3 will receive 9 mg/kg cilnidipine for 3 weeks intragastrically after induction of hypertension by DOCA-salt as group 2.
Results: DOCA-injected albino rats show marked reduction in creatinine clearance. Treatment with cilnidipine improves creatinine clearance compared to the non-treated hypertensive group 2. Additionally, it reduces thiobarbituric acid reactive substance, as an index of lipid peroxidation, with a significant increase in superoxide dismutase enzyme in erythrocyte lysates and kidney catalase enzyme activities.
Conclusion: This study points to the possible beneficial renal protective effects of cilnidipine in hypertensive albino rats injected with DOCA for 2 weeks
2.Introduction
3.Methods
3.1 Drugs
3.2 Animals
3.3 Ethics
3.4 Experimental protocol
4.Data analysis
5. Results
5.1 Effect of cilnidipine treatment on systolic blood pressure (SBP) in rats exposed To DOCA salt-induced hypertension
5.2 Effect of cilnidipine treatment on creatinine clearance in rats exposed to DOCA salt-induced hypertension
5.3 Effect of cilnidipine treatment on TBARS and catalase enzyme activity in kidney homogenates in rats exposed to DOCA salt-induced hypertension
5.4 Effect of cilnidipine treatment on SOD levels in erythrocyte lysates in rats exposed To DOCA salt-induced hypertension
6.Discussion
7.Conclusion
8.Acknowledgment
9.Disclosure
10.References
Key Words
Cilnidipine; DOCA; Renal, Hypertension; Antioxidant; Albino Rats.
Introduction
Cilnidipine is reported to be a calcium channel blocker (CCB) that possesses clinical advantages over other dihydropyridine. It has less influence on heart rate and the autonomic nervous system than nifedipine Retard and causes less tachycardia than nisoldipine in hypertensive patients [13,14]. Cilnidipine, was reported to cause an inhibition of the pressor response induced by acute cold stress in addition to its hypotensive effect when administered to spontaneously hypertensive rats (SHRs) [7].
However, no enough experimental studies have been carried out to investigate whether its possible protective effect to vital organs like kidneys in hypertension against stress could be related to possible antioxidant effects.
Antioxidant enzymes consist of superoxide dismutase (SOD), catalase, and glutathione peroxidase. Dismutation of oxygen free radicals by SOD produces hydrogen peroxide, a more stable reactive oxygen species (ROS) which, in turn, is converted to water by catalase and glutathione peroxidase. An antioxidant effect may result from either activation or mimicry of antioxidant defenses. Alternatively, it may be due to interaction with factors involved in the activation of oxidative stress [20].
Historically, a study has reported that treatment of the endothelium with dihydropyridine calcium antagonists resulted in an increased release of nitrogen monoxide that is not due to a modulation of L-type calcium channels, because macrovascular endothelial cells do not express this channel. Also, The authors explained their findings via an increase in nitrogen monoxide availability that might cause part of the vasodilation, and might contribute to the antithrombotic, antiproliferative, and antiatherosclerotic effects of dihydropyridine calcium antagonists [1].
More recently, Dihydropyridine-based calcium antagonists (DHPs) are widely used drugs for the treatment of hypertension and angina pectoris. Nifedipine, one of the most widely used DHPs, inhibits apoptotic cell death of endothelial cells (ECs) as well as vascular inflammation and subsequently improves endothelial function in patients with cardiovascular risk factors, including hypertension and/or diabetes, thus slowing the development and progression of atherosclerosis in these patients. Several papers have suggested that nifedipine exerts beneficial metabolic effects in vivo through its anti-inflammatory properties as well. However,the underlying molecular mechanisms for the cardiometabolic actions of nifedipine remain to be elucidated, because ECs do not possess voltage-operated L-type calcium channels. Meanwhile, we have very recently found that Bay w 9798, a dihydropyridine structurally related to nifedipine with no calcium antagonistic ability, has anti-oxidative and anti-inflammatory properties in vitro. This means that more studies are needed to investigate the role of oxidative stress in the development of vascular injury, especially focusing on the relationships between advanced glycation end products-receptor system, oxidized low-density lipoprotein and tumor necrosis factor-alpha and vasculopathy. [23].
Amlodipine, being one of the dihydropyridines, influence on the membrane permeability and cholesterol deposition in the membrane, its antioxidative action, stimulation of the NO release, as well extracellular matrix (ECM) regulation are not directly connected to the decrease of the resistance of the blood vessels – antihypertensive action. The cellular and molecular mechanisms of the observed amendment of the endothelium function, delay of the cardiovascular action disturbances as the result of amlodipine use are widely investigated, however up to now are not fully clear and unequivocal [22].
Therefore, the present study investigates, in the DOCA salt-induced hypertension model, the effect of treatment with cilnidipine, as a dihydropyridine CCB, on creatinine clearance, and some oxidative markers, in kidney homogenates of albino rats.
Cilnidipine was provided as powder by Lonsino Medical Products Co., Ltd. China. Super oxide dismutase (RANSOD) was supplied by Randox Laboratories, Kearneysville, WV, USA. All other chemicals were purchased from Sigma Chemicals, St Louis, MO, USA.
Thirty-six male albino rats weighing 180–200 g, were used in this study. They were randomly allocated into three groups of 12. Rats were housed in individual plastic cages and allowed one week to acclimate to their surroundings before the beginning of the experiments. Standard rat chow and tap water were available ad libitum for the duration of the experiments, unless otherwise noted.
All procedures were in accordance with the National Institute of Health’s Guide for the Care and Use of Laboratory Animals, as well as the guidelines of the Animal Welfare Act.
Albino rats were divided into three groups. Group 1 was the control group. Rats of group 2 were injected by Deoxycorticosterone acetate (DOCA) salt sc twice/week + 1% NaCl drunken for 2 weeks to be rendered hypertensive [12]. Rats of group 3 were administered intragastrically with 9 mg/kg/day cilnidipine, dissolved in a standard volume of solution of Arabic Gum [18,19] for 3 weeks intragastrically after induction of hypertension by DOCA-salt as group 2. To exclude any action of solvent on high blood pressure and other parameters: In the pilot study, an experimental group (n = 12) received standard volume of solution of Arabic Gum equal to that used to dissolve cilnidipine in the experimental study to test it without the drug for a duration of 3 weeks ( duration of drug therapy in group 3). Results were similar to that obtained with Group 2 (control hypertensive i.e. no change on the high blood pressure or anti-oxidant parameters→ same results as group 2 [ control hypertensive without treatment]). So no need to write another column with the same results of group 2 [ control hypertensive with solvent]
SBP was measured by a tail-cuff sphygmomanometer (UR-5000,Ueda Co, Ltd, Japan). SBP measurements were conducted in all groups over a period of 8 hours post-injection at 30 min. intervals. For each animal an average of at least three consecutive measurements was taken to reduce variability [3].
Albino rats were individually housed in metabolic cages for 24 hours at the end of the study. During the 24 hour period, animals continued to have free access to tap water and standard laboratory rat chow. Urine volume per 24 hours was calculated for each animal. Serum and urine creatinine concentrations were measured using a Beckman® Analyzer (Beckman Coulter, Brea, CA, USA) according to the picric acid colorimetric method,5 and creatinine clearance in different groups was calculated using the following formula: (urine creatinine(mg/dL) × urine volume per day(mL) ÷ 1440(min)) ÷ serum creatinine(mg/dL)) ÷ body weight(g) × 100. (10).
At the end of the study, blood samples were collected from rats from all groups for measurement of SOD levels in erythrocyte lysates using commercially-available colorimetric assay kits, based on an indirect xanthine-xanthine oxidase method, [6] and results were expressed in IU/mL.
Kidney homogenates were rinsed with cold 0.14 M sodium chloride and homogenized in 25% ice-cold 50 mM Tris-HCl buffer (pH 7.4). 150 μL of the tissue supernatant of samples were diluted to 500 μL with deionized water. 250 μL of 1.34% thiobarbituric acid was added to each tube, followed by the addition of an equal volume of 40% trichloroacetic acid. The mixtures were then shaken and incubated for 30 minutes in a boiling water bath. Tubes were allowed to cool to room temperature, and the absorbance was then read at 532 nm, using zero concentration as blank.
The protein content of kidney homogenates was determined by spectrophotometer according to the method of [2]. The aim is to relate the oxidative marker concentrations to the total tissue protein.
Data analysis
The results are presented as mean ± standard deviation [SD], and evaluated using one-way analysis of variance (ANOVA), followed by Tukey's post hoc determination, using GraphPad Prism (version 3.00; GraphPad Software, La Jolla, CA, USA).
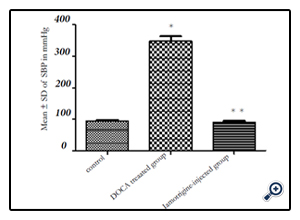
Figure 1 demonstrates a significant (**p < 0.05) decrease in SBP in DOCA salt-treated hypertensive rats treated with cilnidipine [ group 3]. Compared to the control group [1], the DOCA salttreated group (group 2) was associated with a significant (*p <0.05) increase in SBP.
Figure (1).Reduction in high systolic blood pressure of DOCA salt-induced hypertension after intragastric administration of cilnidipine in albino rats treated with DOCA-salt + 1% NaCl to induce hypertension.
Figure (1) A significant (p < 0.05) of DOCA salt-induced hypertension after intragastric administration of cilnidipine in albino rats treated with DOCA-salt + 1% NaCl to induce hypertension.
Notes: *p < 0.05 significant increase in SBP compared to control non-hypertensive group. **p < 0.05 significant decrease in SBP in group (3) compared to DOCA-salt-treated group (2).Abbreviation: SD = standard deviation.
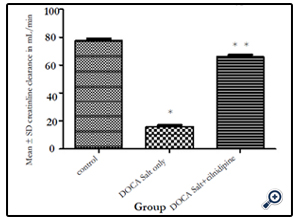
Figure 2 demonstrates an increase in creatinine clearance in DOCA salt-treated hypertensive rats treated with cilnidipine. Creatinine clearance, in mL/min, of the different groups (solution of Arabic Gum, DOCA salt alone, and DOCA salt + cilnidipine) was calculated. Compared to the control group (1), the DOCA salt-treated group (2) was associated with a significant (*p < 0.05) decrease in creatinine clearance. This decrease was significantly (**p < 0.05) increased in cilnidipine group [3].
Figure (2): Improvement of creatinine clearance after intragastric administration of cilnidipine in albino rats treated with DOCA-salt + 1% NaCl to induce hypertension.
Figure (2) Improvement of creatinine clearance after intragastric administration of cilnidipine in albino rats treated with DOCA-salt + 1% NaCl to induce hypertension.
Notes: *p < 0.05 significant reduction in creatinine clearance compared to control group. **p < 0.05 significant increase in creatinine clearance in group (3) compared to DOCA-salt + 1% NaCl to induce hypertension without cilnidipine treatment group (2). Abbreviation: SD = standard deviation.
TBARS levels measured in tested kidney homogenates of the cilnidipine-treated group [3] showed its significant (**p < 0.05) reduction compared to control normotensive and hypertensive levels in groups ( 2 & 3).
Meanwhile, catalase enzyme activity in the homogenates in cilnidpine-treated hypertensive albino rats was significantly (**p < 0.05) increased to almost the control levels of the control normotensive group (1). However, group (2) hypertensive albino rats showed a significant (*p < 0.05) decrease in its activity compared to group normotensive (1) (Table 1).
Table (1). Effect of cilnidipine on TBARS (in nmoL/mg tissue protein) and catalase enzyme activity (in μmoL/min/mg tissue protein) in kidney homogenates.The results are expressed as means ± standard deviation (n = 12 in each group).
The mean ± SD of cilnidipine on SOD levels in erythrocyte lysates.
Exposure to the DOCA salt-induced hypertension model showed a significant (*p < 0.05) decrease in SOD in erythrocyte lysates compared to control group. Concomitant administration of cilnidipine with DOCA salt significantly (**p < 0.05) increased SOD levels compared to control levels (Table 2).
Table (2).Effect of cilnidipine on superoxide dismutase (SOD) level in IU/mL erythrocyte lysates isolated from tested albino rats in the three groups. The results are expressed as means ± standard deviation (SD).
Discussion
The current study was undertaken to determine whether cilnidipine administered to rats with DOCA salt-induced hypertension can protect against oxidative stress associated with hypertension, and can produce a beneficial effect on creatinine clearance compared with rats without cilnidipine treatment.
The increase in TBARS production may contribute to impaired kidney function. Oxidative stress causes hypertrophy of nephrons via increased production of angiotensin II (AII), mediated by reactive oxygen species (ROS) [16 and 8].
An increase in tubular endothelin-1 (ET-1) synthesis is reported in oxidative stress, it may induce fibroblast proliferation, interstitial matrix deposition, and infiltration of inflammatory cells that result in progressive tubulointerstitial fibrosis. Therefore, it seems that inhibition of oxidative stress can significantly retard the progression of renal and vascular complications. [17].
The antioxidant properties of nifedipine, being one of CCBs, are described as being due to either a direct scavenging effect or the preservation of SOD activity. Under controlled experimental conditions, they may inhibit lipid peroxide formation at concentrations present in plasma. This antioxidant activity is found with high lipophilic CCBs when their chemical structure facilitates proton- donating and stabilization mechanisms that quench the free radical reaction [1].
Cilnidipine causes a decrease in aortic endothelin-1 (ET-1) gene expression might be related also to the protection by cilnidipine against the renal ischemic alterations caused by plasma renin activity (PRA) elevation. Prevention of PRA elevation by cilnidipine would suppress excessive AII production by renin of kidney origin,thereby opposing AII-stimulated ET-1 gene overexpression in renal cells [18,19].
Experimental research has explained the mechanism of DOCA salt-induced hypertension in the form of induction of renal oxidative stress, as shown by a reduction in kidney glutathione, and an increase in lipid peroxidation [11,15].
Cilnidipine, an L/N-type calcium channel blocker (CCB), has been reported to have more beneficial effects on proteinuria progression in hypertensive patients than amlodipine, an L-type CCB. The N-type calcium channel blockade that inhibits renal sympathetic nerve activity might reduce glomerular hypertension by facilitating vasodilation of the efferent arterioles. However, the precise mechanism of the renoprotective effect of cilnidipine remains unknown. Because cilnidipine exerted significantly higher antioxidant activity than amlodipine in cultured human mesangial cells, we hypothesized that cilnidipine might exert a renoprotective effect by suppressing oxidative stress. Cilnidipine, but not amlodipine, ameliorated urinary albumin excretion and decreased urinary 8-hydroxy-2'-deoxyguanosine (8-OHdG) and liver-type fatty-acid-binding protein (L-FABP) in the hypertensive patients. Cilnidipine probably exerts a greater renoprotective effect through its antioxidative properties [21].
Calcium channel blockers (CCBs) are reported to regulate glomerular pressure by changing the afferent and efferent arteriole tone. [24] demonstrated that cilnidipine decreased single nephron filtration fraction, glomerular capillary pressure, afferent and efferent arteriole resistance and proteinuria in nitric oxide synthaseinhibited SHR, indicating that cilnidipine attenuated the glomerular hypertension and prevented the proteinuria. Similar arteriole responses were observed in the kidney of dogs [5] and hydronephrotic spontaneous hypertensive rats (SHR) [9]. Vasodilatory effect of cilnidipine, an L-type and N-type calcium channel blocker, on rat kidney glomerular arterioles. Int Heart J.,49:723–732 Importantly, [9] revealed that nifedipine, another L-type CCB, dilated afferent, but not efferent, artery, whereas cilnidipine dilated both afferent and efferent artery, suggesting that N-type calcium channel inhibition by cilnidipine might elicit efferent vasodilation. Therefore, it can be expected that the effect of cilnidipine on glomerular hemodynamics explains part of the renoprotective effect of cilnidipine in SHR/ND in the present study. However,[5] reported that the effects of cilnidipine and amlodipine on the efferent/afferent ratio arteriole dilation were similar in hydronephrotic kidney. Taken together, it seems likely that cilnidipine elicits renoprotectiveeffect by regulating glomerular hemodynamics.
More animal studies are needed to be addressed to these issues and further in-vitro studies are needed to clarify the precise mechanisms by which the observed anti-oxidant and nephroprotective effects are elicited.
Conclusion
In conclusion, the tested dihydropyridine CCB cilnidipine could protect albino rats against the impairment of kidney function evoked by hypertension induced by DOCA salt injection for 2 weeks, and this may contribute to the beneficial effect against end-organ damage and oxidative stress reported in clinical trials. Further investigations on cilnidipine are needed to discover their beneficial role in protection or management of renal diseases.
Acknowledgments
This research was officially supported by the Medical Research Service of the Ain Shams University. It was financially supported by the laboratory of the Pharmacology Department, Faculty of Medicine, Ain Shams University.
Disclosure
The author reports no conflicts of interest in this work
References
- Berkels R, Egink G, Marsen T, Bartels H, Roesen R, et al.(2001) Nifedipine increases endothelial nitric oxide bioavailability by antioxidative mechanisms. Hypertension., 37:240–245.
- Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. , 72:248–254.
- Bunag R (1973) Validation in awake rats of a tail-cuff method for measuring systolic pressure. J. Appl. Physiol., 34: 279–282.
- Gutteridge J, Quinlan G (1983) Malondialdehyde formation from lipid Peroxides in the thiobarbituric acid test: the role of lipid radicals, iron salt and metal chelators. J Appl Biochem., 5(4–5):293–299.
- Hayashi K, Wakino S, Sugano N, Ozawa Y, Homma K, et al. (2007) Ca2+ channel subtypes and pharmacology in the kidney. Circ Res., 100:342–353.44.
- Halliwell B, Chirico S (1993) Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr., 57:715S–725S.
- Hosono M, Hiruma T, Watanabe K (1995) Inhibitory effect of cilnidipine on pressor response to acute cold stress in spontaneously hypertensive rats. Jpn J Pharmacol., 69:119–125.
- Hwang J, Ing M, Salazar A (2003) Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ Res., 93:1225–1232.
- Konno Y,Kimura K (2008) Vasodilatory effect of cilnidipine, an L-type and N-type calcium channel blocker, on rat kidney glomerular arterioles. Int Heart J.,49:723–732
- Lu J, Bankovic-Calic N, Ogborn M, Saboorian M, Aukema H (2003). Detrimental effects of a high fat diet in early renal injury are ameliorated by fish oil in Han:SPRD-cy Rats. J Nutr., 133:180–186.
- Maldonado P, Barrera D, Madina-Campos O, Hernandez-Pando R, Ibarra- Rubio M, et al. (2003) Aged garlic extract attenuates gentamycin induced renal damage and oxidative stress. Life Sci., 20:2543–2556.
- Matsumura Y, Hashimoto N, Taira S, Kuro T, Kitano R, et al.(1999) Different Contributions of Endothelin-A and Endothelin-B Receptors in the Pathogenesis of Deoxycorticosterone Acetate–Salt–Induced Hypertension in Rats. Hypertension. ;33:759-765
- Minami J, Ishimitsu T, Higashi T, Numabe A, Matsuoka H (1998) Comparison between cilnidipine and nisoldipine with respect to effects on blood pressure and heart rate in hypertensive patients. Hypertens Res., 21:215–219.
- Minami J, Ishimitsu T, Kawano Y, Numabe A, Matsuoka H (1998) Comparison of 24-hour blood pressure, heart rate, and autonomic nerve activity in hypertensive patients treated with cilnidipine or nifedipine retard. J Cardiovasc Pharmacol., 32:331–336.
- Mohamadin A, Abdou S, El-Shafey M, El-Tawil O, Moneir S (2003) Protective effect of inosine against gentamycin induced oxidative stress and nephrotoxicity in rats. J Biol Ph Sci.,1:24–37.
- Nakazono K, Watanabe N, Matsuno K, Sasaki J, Sato T (1991) Inoue M. Does superoxide underlie the pathogenesis of hypertension?. Proc Natl Acad Sci U S A., 88(22):10045–10048.
- Orth S, Esslinger J, Amann K, Schwarz U, Raschack M, et al. (1998) Nephroprotection of an ETA-receptor blocker (LU 135252) in salt-loaded uninephrectomized stroke-prone spontaneously hypertensive rats. Hypertension., 31:995–1001.
- Sakaki T, Naruse H, Masai M, Takahashi K, Ohyanagi M, et al.(2003) Cilnidipine as an agent to lower blood pressure without sympathetic nervous activation as demonstrated by iodine-123 metaiodobenzylguanidine imaging in rat hearts. Ann. Nucl. Med., 17 (4): 321–326.
- Sakaki T, Naruse H, Masai M, Takahashi K, Ohyanagi M, et al.(2003) Cilnidipine as an agent to lower blood pressure without sympathetic nervous activation as demonstrated by iodine-123 metaiodobenzylguanidine imaging in rat hearts. Annals of Nuclear Medicine 17 (4): 321–326.
- Sato J, O’Brien T, Katusic Z, Fu A, Nygren J, et al. (2002) Dietary antioxidants preserve endothelium dependent vasorelaxation in overfed rats. Atherosclerosis., 161:327–333
- Soeki T, Kitani M, Kusunose K, Yagi S, Taketani Y, et al.(2012) Renoprotective and antioxidant effects of cilnidipine in hypertensive patients. Hypertens Res. 2012 Nov;35(11):1058-1062.
- Vitolina R, Krauze A, Duburs G, Velena A. (2012) Aspects of The Amlodipine Pleiotropy in Biochemistry, Pharmacology and Clinics. IJPSR., 3(5): 1215-1232
- Yamagishi S, Nakamura K, Matsui T. (2008) Role of oxidative stress in the development of vascular injury and its therapeutic intervention by nifedipine. Curr Med Chem., 15(2):172-177.
- Zhou X, Ono H, Ono Y, Frohlich ED. (2002) N- and L-type calcium channel antagonist improves glomerular dynamics, reverses severe nephrosclerosis, and inhibits apoptosis and proliferation in an l-NAME/SHR model. J Hypertens., 20:993–1000