UVB Effects on Microalgae Production: Implication to the Outdoor System
Taguchi S*
Hokkaido National Fisheries Research Institute, Kushiro and National Institute of Polar Research, Midori Cho, Tachikawa, Tokyo, Japan.
*Corresponding Author
Satoru Taguchi,
Hokkaido National Fisheries Research Institute,
Kushiro and National Institute of Polar Research 10-3 Midori Cho, Tachikawa, Tokyo, Japan.
Tel: 42-656-4668
E-mail: satoru.sio@gmail.com
Received: November 23, 2017; Accepted: January 10, 2018; Published: January 19, 2018
Citation: Taguchi S. UVB Effects on Microalgae Production: Implication to the Outdoor System. Int J Marine Sci Ocean Technol. 2018;5(1):95-102.
Copyright: Taguchi S© 2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Responses of the green algae Tetraselmis suecica (Kylin) Butcher to ultraviolet-B (UVB) radiation were determined for 150 days. Vegetative cells were incubated under a 10-h light:14-h dark cycle of photosynthetically active radiation (PAR) with UVB (inhibitory phase) and PAR without UVB (recovery phase). During the inhibitory phase, cells ceased swimming, became bleached, and showed a decrease in population size in terms of the cell density, the concentration of Chlorophyll a (Chla) and organic carbon (C) as well as 13C assimilation. Under PAR + UVB radiation, the vegetative cells transformed to resting cells. When UVB radiation was removed, resting cells regained active swimming, vegetative conditions and showed an immediate increase in cell density. In outdoor, large scale incubations, microalgae might be damaged by UVB radiation and transformed to resting cells, but regain a vegetative condition when UVB radiation is removed.
2.Introduction
3.Materials and Methods
4.Results
4.1 Cellular Responses to UVB
4.2 C/Chla Ratio
4.3 Carbon Assimilation
5.Discussion
5.1 UVB Exposure
5.2 C/Chla Ratio.
5.3 Biotechnological Implications
6.Acknowledgments
7.References
Keywords
Bleaching; Carbon/Chlorophyll a Ratio; Cell Density; Photosynthetically Active Radiation.
Introduction
The effects of ultraviolet-B (UVB) radiation have been studied on several species of marine microalgae [e.g., [1-4]]. These studies showed diverse deleterious effects of UVB radiation on the growth of microalgae, suggesting that cellular DNA repair mechanisms in dark and light processes [5] cannot keep pace with the damage rate even under solar UVB radiation. The effects of the nutrient supply on DNA damage by UVB in phytoplankton should also be considered to understand the DNA damage and repair mechanisms of phytoplankton [6, 7]. Cells may adapt by developing repair or tolerance mechanisms to counteract UVR damage as long asthey are vegetative [8]. Long-term experiments should be employed to study the balance between DNA damage and repair without nutrient limitation.
In shallow outdoor ponds lit with UVR, particularly in the midlatitude regions during summer, microalgae are continuously exposed to UVB as natural assemblages of phytoplankton [9] and eventually decrease in the population size because of the mortality caused by UVB [10]. Even in shallow outdoor ponds, the rate of the vertical mixing of water, including cells, should be considered [11] in relation to the effective UVB penetration depth, which is 10% of UVB at the surface [10] defined the UVB photoactive zone. In addition to the UVB photoactive zone, the biologically effective UVB day length relative to PAR should be considered [12]. The biologically effective UVB day length in surface water is reduced by approximately 25 % compared to those in air.
To simulate inhibition and recovery in the growth under UVB radiation, long-term incubation experiments should be conducted [13]. Most studies concentrate on short-term incubation experiments, which only induce vegetative cell stages, including actively dividing cells and senescent cells. Few studies address long-term exposures of cells to UVB radiation to extend the duration of exposure experiments to observe what would happen to UVB-damaged cells. When long-term doses of UVB are provided, the unicellular population starts to lose vegetative cells and eventually population sizes were significantly reduced. Programmed cell death might be induced as a result in the loss of vegetative conditions as observed in unicellular microalgae, including chlorophytes [14-18]. Adaptive vegetative cells also transformed into resting cells, which was defined based on morphology and metabolic activity [19], for the survival of the population.
In the present study, a long-term UVB exposure experiments were employed to study inhibition and the survival of the green algae Tetraselmis suecica (Kylin) But cherto primarily determine how cells transform to resting cells and recover from UVB damage. Tetraselmis suecica are among the most ubiquitous eukaryotic organisms [20] and have extraordinarily high tolerance to salinity, temperature, nutrient limitation, and irradiance under systematic vertical mixing [11, 21, 22]. Tetraselmis suecica grows as single, motile cells that are visible under a light microscope, reaching a concentration of over ten million cells per milliliter [11]. These features make the microalgae preferable candidates to study responses to environmental stress, such as responses to UVB [23]. Tetraselmis suecica is often used as an experimental species [24] since this species is considered to be a descendant of primitive species [25], which appeared on the Earth when stronger UVB radiation was present than currently observed [26]. Tetraselmis suecica has been identified to be the most tolerant to UVB based on the relative inhibition experiments over 0.4 W m-2 of UVB irradiance as well as able to acclimate quickly to UVB conditions among 7 marine microalgae [27]. The aim of the present study was to elucidate how cells response to UVB radiation and determine how cells recover from UVB damage when UVB radiation was removed. Inhibition of cellular response was monitored under two intervals between dilutions; 1 or 2 weeks because cell growth of Tetraselmis suecica depended on the interval between dilutions in a shallow outdoor flume [11].
Materials and Methods
The unicellular marine chlorophyte used in the present study was Tetraselmis suecica (Kylin) Butcher obtained from the culture collection of Hokkaido National Fisheries Research Institute, Japan. The cultures were initiated from the inoculum of a late log-phase of stock cultures.
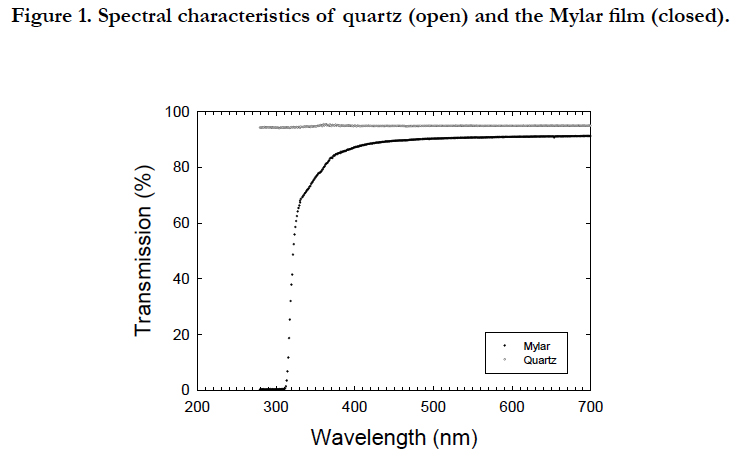
Vegetative cells were grown under a 10-h light:14-h dark cycle at 100 μE m-2 s-1 of PAR at the culture surface provided by coolwhite fluorescence tubes (model Toshiba, Tokyo, Japan) in 12 UVB radiation transparent quartz bottles (250 mL) with a small Teflon-coated magnetic stir.
All cultures were placed on a multiple magnetic stirrer (HS-360, Asone, Osaka, Japan) to obtain an even distribution of cells within the bottle. The inhibitory phase with UVB radiation and recovery phase without UVB radiation were prepared for the period from 32 to 59 and 79 to 115 in Julian days and for the period from 59 to 79 and 115 to 150 in Julian days, respectively. The photon flux density was measured using a spherical quantum sensor (Model GSL 100, Biospherical Instrument, San Diego, USA). UVB radiation was provided from a Mylar filter (Figure. 1), which cut the wavelength shorter than the 280 nm wavelength provided by the ultraviolet fluorescent bulbs (FL20SE, Phillips, Pennsylvania, USA). Coolwhite fluorescence and ultraviolet fluorescent tubes provided an irradiance of 1.2 mW cm-2 of PAR and 19 μW cm-2 of UVR. Since the filters were aged and the light penetration characteristics deteriorated [28], the filters were replaced every week during the UVB exposure experiments. Ultraviolet radiation was determined using ultraviolet radiometers (UVX-25, Funakoshi, Tokyo, Japan), with a peak sensitivity at 318 nm. Exposure experiments were conducted at 32 ± 1°C in a temperature controlled room. The duration of the first and second UVB exposures were 28 and 37 days, respectively. All cultures were grown in filter-sterilized (0.2 μm pore size) seawater collected 200 km southward offshore of Hokkaido and enriched with the nutrients of f/2 medium [29].
The culture was diluted by 25 % in volume with fresh f/2 medium. This dilution reduced the potential destruction of dissolved organic matter due to UV radiation [30].
Samples were obtained before and after dilution for an entire period. Growth was followed by weekly measurements of the cell density and Chla concentration. The cell density was examined using a hematocytometer for densities higher than 107 cells mL-1 or a settling chamber (50 mL, Zeiss, Germany) for densities lower than 107 cells mL-1. The concentration of Chla was determined using the fluorometric method according to [31] using a fluorometer (Model 10, Turner Design, San Diego, USA) with consideration of chlorophyll b [32].
The samples for the particulate organic carbon (C) analyses and 13C measurements were obtained in the second PAR +UVR exposure experiment. Samples for C analyses were collected on glass fiber filters (GF/C, Whatman, UK) that were pre-combusted for 6 h at 500°C to remove any contaminating carbon. The samples on the filters were analyzed using an elemental analyzer (Model MT-5 CHN, Yanaco, Japan). Reflecting the limitation of the sample size, cellular nitrogen was not determined. The samples for the measurements for 13C assimilation index were incubated for 6 h with 0.5 mL of 13C-sodium bicarbonate (0.1 g L-1) on the day of dilution to yield a final concentration of 0.05 mg L-1, although a caution should be paid due to a week-long incubation. The isotopic ratios of 13C and 12C were determined via infrared absorption spectrometry [33] using a 13C analyzer (Model 1000, Nihon Bunko, Tokyo, Japan). The assimilation rate was calculated according to the method [34].
Data in the transition between the inhibitory and recovery phases were included to each regression analysis due to difficulty in distinguishing data points in the transition.
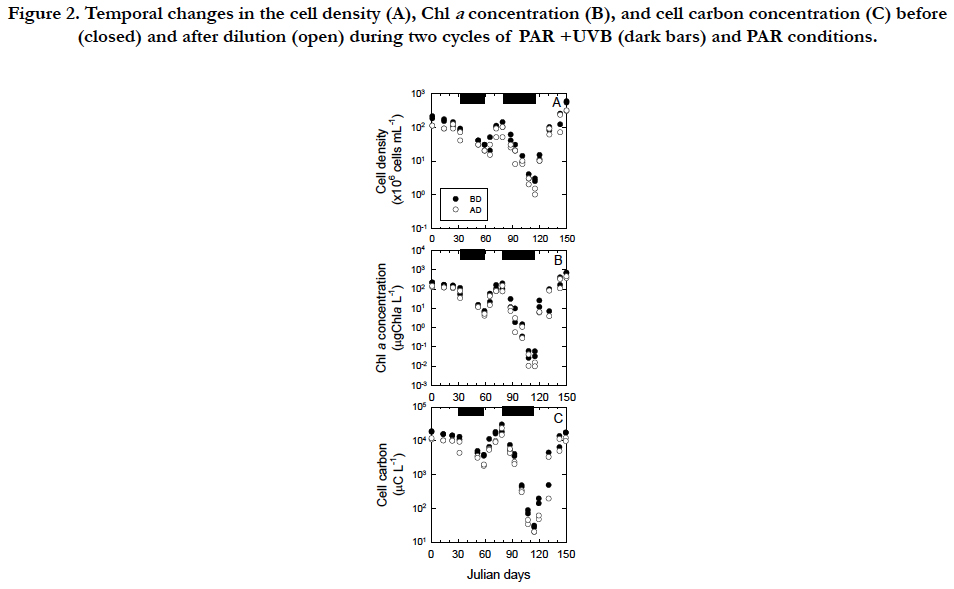
Temporal changes in the cell density, Chla concentration, and cell C of Tetraselmis suecica were similar between the first and second cycles of the PAR+UVB(inhibitory phases) and PAR (recovery phases) conditions during the 150day-exposure experiments (Figure. 2). The temporal changes were characterized by an inhibitory phase (32 to 59 and 79 to 115 in Julian days) with UVB radiation and recovery phase(59 to 79 and 115 to 150 in Julian days) without UVB radiation. Under the PAR+UVB condition, the cell density before dilution decreased in both inhibitory phases at rates of −0.017 d-1 and −0.048 d-1 and reached 10-3 % and10-6% of the initial cell density of 2.0 x 108 cells mL-1, respectively (Figure. 2A). The decrease in the second phase was approximately 2.8 times faster than that in the first phase (Figure. 2A). Under the PAR+UVB condition, the Chla concentrations decreased in both inhibitory phases at rates of −0.038 d-1 and −0.099 d-1 and reached 1% and 0.01% of the initial Chla concentrations of 2.1 x 102 μg Chla L-1, respectively (Figure. 2B). The decrease in the second phase was approximately 2.6 times faster than that in the first phase (Figure. 2B). Under the PAR + UVB condition, the C concentrations decreased in both inhibitory phases at rates of -0.019 d-1 and -0.086 d-1 and reached 10 % and 0.1 % of the initial C concentrations of 1.8 x 104 μg C L-1, respectively (Figure. 2C). The decrease in the second phase was approximately 4.5 times faster than that in the first phase (Figure. 2C). Although healthy vegetable cells swim with four flagella, UVB-exposed cells turned white due to an apparent lack of chlorophyll pigments and were also completely still because they were observed to lack the four flagella via microscope observation. The degree of decrease in the Chla and C concentrations was 104-fold, whereas the degree of decrease in cell density was 102-fold due to relatively resistant, slow cell disintegration. When UVB radiation was removed, the cell densities recovered to those of healthy vegetable cells under the PAR conditions (Figure. 2A). Under the first and second PAR conditions, the cell density increased immediately and the increase in the second phase was approximately 3.6 times faster than that in the first phase. Under the PAR condition, the Chla concentration immediately increased in both recovery phases and the increase in the second phase was approximately 2.8 times faster than that in the first phase (Figure. 2B). Under the PAR condition, the C concentration immediately increased in both recovery phases at rates of 0.040 d-1 and 0.078 d-1, respectively, and the increase in the second phase was approximately 1.9 times faster than that in the first phase (Figure. 2C).
Figure 2. Temporal changes in the cell density (A), Chl a concentration (B), and cell carbon concentration (C) before (closed) and after dilution (open) during two cycles of PAR +UVB (dark bars) and PAR conditions.
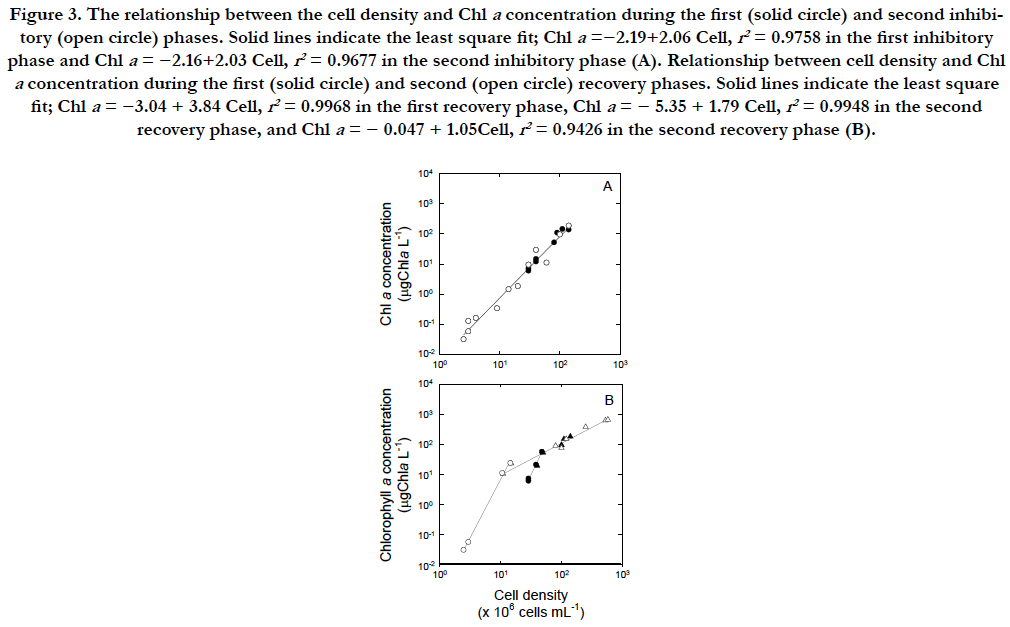
When the inhibitory and recovery rates were compared, the increase of the Chla concentration was approximately 2 times faster than that of the cell density. The inhibitory and recovery rates were highest for the Chla concentration, indicating that this parameter was most sensitive to UVB exposure among the three parameters. The rate of change in cellular Chla contents was1.03 ± 0.6 ng Chla cell-1 during the steady state (1 to 32 in Julian days). The rates during the inhibitory phases (2.06 ± 0.3 ng Chla cell-1) were significantly higher than those during the steady state (Figure. 3A). When UVB radiation was removed, the cells became vegetative through two steps in each recovery phase (Figure. 3B). The first step was approximately 4 times steeper than the second step of each recovery phase although the first steps were similar between the two recovery phases (4.2 versus 3.8ng Chla cell-1 in closed and open circles) as the second steps were similar (~1.1 ng Chla cell-1 in closed and open triangles). The rate during the second step of presumed recovery phase was 1.05 ± 0.3 ng Chla cell-1.
Figure 3. The relationship between the cell density and Chl a concentration during the first (solid circle) and second inhibitory (open circle) phases. Solid lines indicate the least square fit; Chla =−2.19+2.06 Cell, r2 = 0.9758 in the first inhibitory phase and Chl a = −2.16+2.03 Cell, r2 = 0.9677 in the second inhibitory phase (A). Relationship between cell density and Chl a concentration during the first (solid circle) and second (open circle) recovery phases. Solid lines indicate the least square fit; Chl a = −3.04 + 3.84 Cell, r2 = 0.9968 in the first recovery phase, Chl a = − 5.35 + 1.79 Cell, r2 = 0.9948 in the second recovery phase, and Chl a = − 0.047 + 1.05Cell, r2 = 0.9426 in the second recovery phase (B).
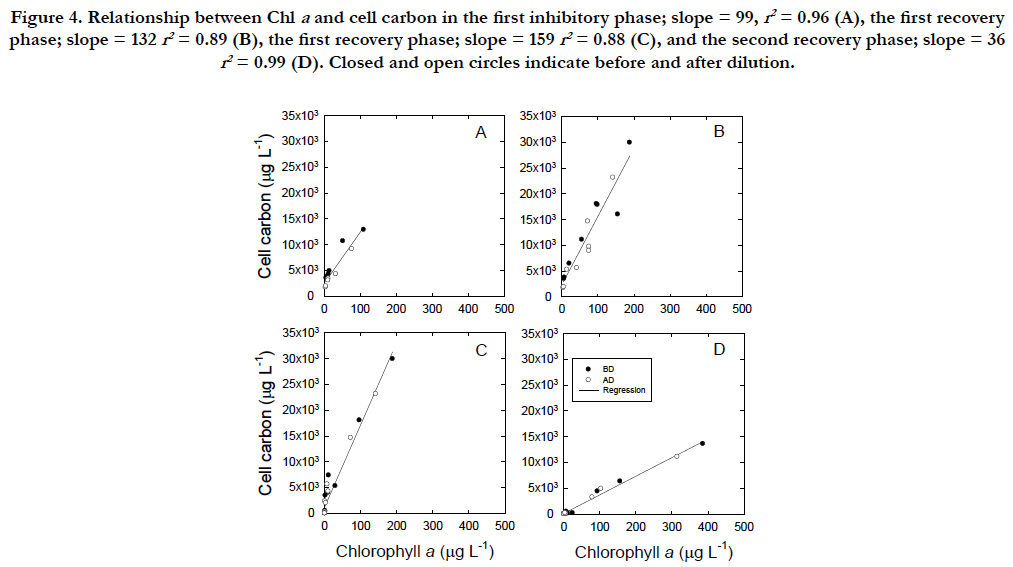
The C/Chla ratios varied not only in the inhibitory (Figure. 4A, C) and recovery phases (Figure. 4B, D) but also in two inhibitory to- recovery cycles (Figure. 4A, B versus Figure. 4C, D). During the first 3 weeks prior to the cycles, the C/Chla ratio at the 95% confidence limit was 70.0 ± 8.3μgC(μgChla)-1. During the first cycle, the ratios were not significantly different between 99.3 ± 13μgC(μgChla)-1 during the first inhibitory phase and 132 ± 31aμgC(μgChla)-1 during the first recovery phase (p > 0.01, Figure.4A, B), whereas those decreased significantly from 159 ± 31μgC(μgChla)-1 in the second inhibitory phase to 36 ± 13μgC(μgChla)-1 in the second recovery phase (Figure.4C, D). The first inhibition rate of the Chla concentration was 2 times higher than that of the C concentration (-0.038 d-1 versus -0.019 d-1, Figure.2B, C), whereas the second inhibition rate of the Chla and C concentrations were similar (−0.099 d-1 versus −0.086 d-1).
Figure 4. Relationship between Chl a and cell carbon in the first inhibitory phase; slope = 99, r2 = 0.96 (A), the first recovery phase; slope = 132 r2 = 0.89 (B), the first recovery phase; slope = 159 r2 = 0.88 (C), and the second recovery phase; slope = 36 r2 = 0.99 (D). Closed and open circles indicate before and after dilution.
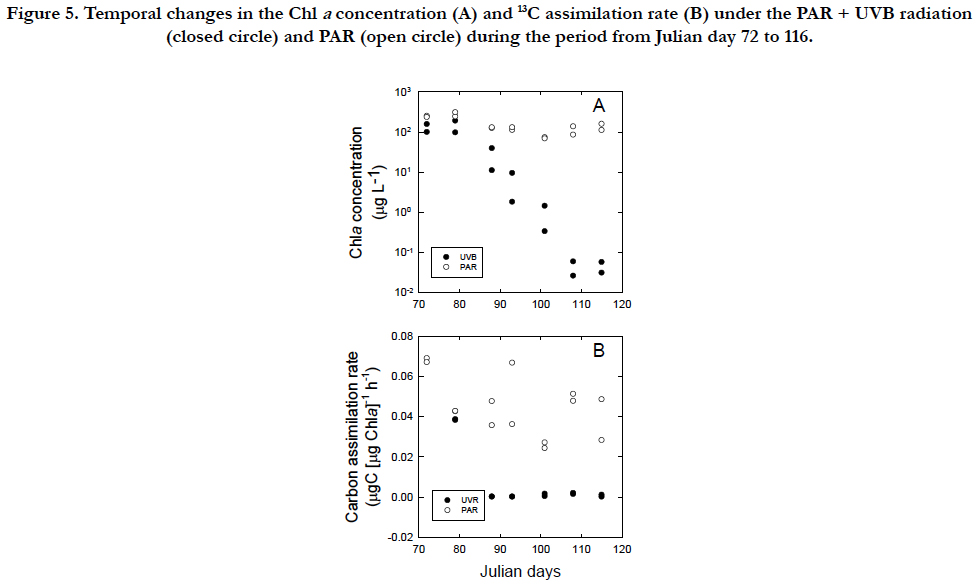
The carbon assimilation rate of healthy vegetable cells under the PAR condition was 0.068μgC(μgChla)-1 h-1 at the concentration of 142μgChlaL-1 (Figure. 5A, B). In the PAR +UVR conditions, the Chla concentration decreased from 106μgChla L-1 on 77 in Julian day to 0.122 μgChla L-1 on 107 in Julian day (Figure. 5A) and the cells lost their swimming ability and bleached to a white color under the microscope, whereas when UVB radiation was removed, the Chla concentration remained at a similar level 153 ± 74 μgChla L-1 until 115 in Julian day and the cells maintained their swimming ability and showed the green color of vegetable cells. The carbon assimilation rate of cells under the PAR +UVB condition decreased sharply and was nearly undetected after Julian day 87, whereas the rate under the PAR condition remained at levels 0.0415 ± 0.0121μgC(μgChla)-1 h-1 similar to those of vegetable cells throughout the incubation period (Figure. 5B).
Figure 5. Temporal changes in the Chl a concentration (A) and 13C assimilation rate (B) under the PAR + UVB radiation (closed circle) and PAR (open circle) during the period from Julian day 72 to 116.
Solar ultraviolet radiation deteriorates the growth of microalgal cells [e.g., [1, 35]], whereas micro algal cells not only have the capacity to synthesize UVB-protective pigments [36] but also to repair DNA damage induced by UVB through both dark and light repair mechanisms [e.g., [37, 38]]. Exposure experiments with light-dark cycles should be conducted for a long enough period of time to simulate the response of cells under natural light conditions. However, long-term exposures may lead to a false interpretation of UVB damage on cells due to changes in optical characteristics from the aging of UV-cutting filters [28] and photo degradation of dissolved organic matter (DOM) in enriched f/2 medium from exposure to UVB [30]. In the present study, the optical characteristics were maintained by replacing new Mylar filters every week. The photo degradation of inorganic nutrients, such as phosphate [39] and chelating agents, such as EDTA, occurred in f/2 medium during the 150-day incubation according to [40]. Dilution with a new medium, as employed in the present study, ensured the reduction in photo degradation of DOM from exposure to UVB and also little nutrient limitation. The UVB radiation (19 μW cm-2) in the present study was approximately similar to that which led to a reduction in the Chla but an increase in intercellular carotenoids of the same species in the short-term experiments [23].
The first noticeable response to UVB radiation was the loss of photosynthetic pigments, such as Chla, and loss of swimming ability, as observed by [41], but not [18, 22] Although healthy vegetative cells swim with four flagella, UVB-exposed cells turned white in color, reflecting an apparent lack of chlorophyll pigments, and were also completely still as they were observed to lack the four flagella under the microscope observation. However, the Chla contents were never completely lost (Figure. 6A), as observed by [18]. The increase in the cell volume induced by UVB exposure was attributed to DNA damage and the consequent cessation of the processes involved in the cell cycle [42, 43]. During the increase in the cell volume, energy is accumulated in the cell. The accumulated energy could be advantageous for cells if the cause of the stress disappears. However, as the stress continued, de novo synthesis of cellular components required for cell growth and maintenance was obstructed, leading to resting cell. Resting cell may not occur in the whole population at once, but was observed in a partial population. The faster values in the second inhibitory phase may suggest that there are cumulative effects of UVB on cell as known for Rhodomonas salina, Chaetocerossp. And Isochrysis galbana [27]. The inhibitory rate of cells was -0.0173 d-1 (Figure. 2A), whereas the destruction rates of Chla was -0.0382 d-1 (Figure. 2B) which was 2 times faster than those cell numbers. UVR did not cause cell death, confirming the previous studies [18, 22], although cell death was reported by [44].
The results of the present experiments under an artificial UVB light source should be compared with studies conducted in shallow outdoor flumes under natural solar radiation [11, 45]. Although Tetraselmis suecica can grow exponentially during each 4-day dilution with a dilution rate of 50 % [11, 45], long dilution periods of 7- or 14-day dilutions with a dilution rate of 25% were employed in the present study. The cell density was approximately 2 x 106 cells mL-1 after dilution in outdoor race way [11, 45], whereas higher cell densities of 100 ~ 1 x 106 cells mL-1 were maintained under the PAR condition in the present study. Cessation of swimming and almost completeble aching of cellular pigments indicate that the long exposure of UVB radiation in the present study was more influential in the smaller containers than the shallow outdoor flume. However, the UVB damaged resting cells recovered to normal vegetative cells when the UVB radiation was lifted. By switching life styles, T.suecica can survive a dose of UVB radiation, which has also been observed in Antarctic species of Chaetoceros under UVB radiation [35]. Under natural solar conditions, some species may have the ability to repair DNA damaged resulting from UVB through both dark and light repair mechanisms [37]. Under PAR +UVR conditions, the slopes of the regression relationship between the cell density and Chla concentration were similar between the two inhibitory phases (Figure. 3A).When UVB radiation as removed, the cells became vegetative by over whelming synthesis of Chla pigments during the first step of recovery phase (Figure. 3B). The synthesis rate of Chla in relation to the cell density during the second step of recovery phase was similar to that during the normal growth period (1 to 32 Julian days).
The synthesis of Chla in relation to cell C follows a linear relationship, regardless of the inhibitory and recovery phases (Figure. 4A). The smallest C/Chla ratio is provided by the maximum rate of Chla synthesis under PAR conditions (Figure. 4D) with the effect of self-shading [46] in addition to a packaging effect [47]. The rate of Chla loss may be highest in the cellularcompounds, when bleaching occurs through UVB radiation.The C/Chla ratio of healthy vegetative Tetraselmis suecica ranged from 30 to 50 μgC(μgChla)-1 [45]. An increase in the C/Chla ratio from healthy values to 99μgC(μgChla)-1 during the first cell inhibitory phase indicated that UVB arrested the synthesis of Chla. A successive increase in the C/Chla ratio to 159 μgC(μgChla)-1 during the second inhibitory phase suggests that UVB damage may have accumulated within the cells. The 13C assimilation at the end of the second inhibitory phase was negligible (Figure. 5B), although it should be interpreted cautiously because of a relatively long incubation with 13C. However, the cells could have recovered to synthesize Chla when UVB radiation was removed (Figure. 5A). The finding may indicate that the cells of T.suecica experienced a resting stage under severe environmental stresses, such as high light, potentially including UVB radiation [19]. Cells at the resting stage might possess higher C/Chla ratios than vegetative cells, as indicated in resting cells and resting spores of diatom [19]. The recovery of the C/Chla ratio from 123~145 μgC(μgChla)-1 during the first recovery phase to 36.1 μgC(μgChla)-1 during the second recovery phase may suggest that the first recovery occurred with little acclimation to PAR, whereas the secondary recovery occurred with strong acclimation to PAR. Therefore, the synthesis rate of Chla in relation to the C concentration during the second recovery phase (36.1 ± 13 μgC[μgChla]-1) was approximately half of 70 ± 8.3μgC [μgChla]-1 during the growth period (1 to 32 in Julian days). Changes in both morphology and metabolic activity might confirm that vegetative cells were transformed to resting cells as defined by [19]. When UVB radiation was removed from UVB-damaged resting cells, cells with low Chla contents and little 13C assimilation recovered to healthy, vegetative cells under PAR conditions (Figure. 5B). Minimum Chla contents may be required to recover from resting cells to vegetative cells. Microalgal cells may have two processes in relation to repair from damage by UVB radiation. The cells are photo-activated, and dark enzymatic processes can repair or eliminate the structural changes in macromolecules initiated by UVB radiation [5]. The ability of microalgal cells to survive UVB radiation in the outdoor environment is dependent upon not only the exposure duration and flux of UVB radiation but also on the relative contribution of UVB radiation to PAR [27, 48]. The former and latter radiations are involved in damaging processes and photo-repair mechanisms, respectively. In fact, changes in the contribution of UVB radiation to PAR are significantly variable in the natural marine environment [49].
Outdoor pond or raceway has been employed for mass culture of microalgae for decades [e.g., [50]]. Although mass cultures have been successful for a certain period of duration, crush of cultures often occurs by several reasons. One of the reasons is the effects of UVB on the culture in a shallow pond although it has been remained unresolved in the fields of biotechnology than algal physiology or ecology. When cultures experience crushed, microscopic observation or physiological examination by using chlorophyll fluorescence [e.g., [51, 52]] should be conducted to examine if cells are UVB damaged or not. When the UVB damage was suspicious, the present study recommends that shielding outdoor ponds from the UVB radiation might be considered.
Acknowledgements
This work was financially supported in part through a grant from the National Institute of Environmental Science (1993-3 [3]). Author was grateful for K. Sasaki and T. Shimoda at the National Institute of Fisheries Sciences for the analysis of 13C samples. The laboratory assistance of T. Konuki was appreciated.
References
- Jokiel PL, York RH. Importance of ultraviolet radiation in photoinhibition of microalgal growth. Limnology and Oceanography. 1984 Jan 1;29(1):192- 8.
- Döhler G. Influence of UV-B (290-320 nm) radiation on photosynthetic 14CO2 fixation of Thalassiosira rotula Meunier. Biochemie und Physiologie der Pflanzen. 1989 Jan 1;185(3-4):221-6.
- Cullen JJ, Lesser MP. Inhibition of photosynthesis by ultraviolet radiation as a function of dose and dosage rate: results for a marine diatom. Marine Biology. 1991 Jun 1;111(2):183-90.
- Buma AG, Van Oijen T, Van De Poll W, Veldhuis MJ, Gieskes WW. The sensitivity of Emiliania huxleyi (Prymnesiophyceae) to ultraviolet‐b radiation. Journal of Phycology. 2000 Apr 1;36(2):296-303.
- Friedberg EC. DNA repair. Freeman WH, Co. New York; 1985.
- Döhler G, Hagmeier E, Grigoleit E, Krause KD. Impact of solar UV radiation on uptake of 15N-ammonia and 15N-nitrate by marine diatoms and natural phytoplankton. Biochemie und Physiologie der Pflanzen. 1991 Jan 1;187(4):293-303.
- Timmermans KR, Veldhuis MJ, Brussaard CP. Cell death in three marine diatom species in response to different irradiance levels, silicate, or iron concentrations. Aquatic microbial ecology. 2007 Mar 13;46(3):253-61.
- Nina Bouchard J, Campbell DA, Roy S. Effects of UV‐B radiation on the D1 protein repair cycle of natural phytoplankton communities from three latitudes (Canada, Brazil, and Argentina). Journal of Phycology. 2005 Apr 1;41(2):273-86.
- Kuwahara VS, Toda T, Hamasaki K, Kikuchi T, Taguchi S. Variability in the relative penetration of ultraviolet radiation to photosynthetically available radiation in temperate coastal waters, Japan. Journal of oceanography. 2000 Aug 1;56(4):399-408.
- Neale PJ, Helbling EW, Zagarese HE. Modulation of UVR exposure and effects by vertical mixing and advection. UV effects in aquatic organisms and ecosystems. 2003;1:109-135.
- Laws EA, TaguchiS, Hirata J, Pang L. High algal production rates achieved in a shallow outdoor flume. Biotechnol Bioeng. 1986 Feb;28(2):191-7. Pub- Med PMID: 18555314.
- Kuwahara VS, Taguchi S. Estimating the diurnal cycle and daily insolation of ultraviolet and phototsynthetically active radiation at the sea surface. Photochem Photobiol. 2015 Sep-Oct;91(5):1103-11. PubMed PMID: 26031560.
- Xiong F, Nedbal L, Neori A. Assessment of UV-B sensitivity of photosynthetic apparatus among microalgae: short-term laboratory screening versus long-term outdoor exposure. Journal of plant physiology. 1999 Jul 1;155(1):54-62.
- SegoviaM, Haramaty L, Berges JA, Falkowski PG. Cell death in the unicellular chlorophyte Dunaliellatertiolecta: a hypothesis on the evolution of apotosis in higher plants and metazoans. Plant Physiol. 2003 May;132(1):99-105. PubMed Central PMCID: PMC166956.
- Franklin DJ, Brussaard CP, Berges JA. What is the role and nature of programmed cell death in phytoplankton ecology?. European Journal of Phycology. 2006 Feb 1;41(1):1-4.
- Darehshouri A, Affenzeller M, Lutz-Meindl U. Cell death upon H2O2 induction in the unicellular green alga Micrasterias. Plant Biol (Stuttg). 2008 Nov;10(6):732-45. PubMed PMID: 18950431. PubMed Central PMCID: PMC2923030.
- Zuppini A, Gerotto C, Baldan B. Programmed cell death and adaptation: two different types of abiotic stress response in a unicellular chlorophyte. Plant Cell Physiol. 2010 Jun;51(6):884-95. PubMed PMID: 20457671.
- Garcia-Gomez C, Parages ML, Jimenez C, Palma A, Mata MT, Segovia M. Cell survival after UV radiation stress in the unicellular chlorophyte- Dunaliellatertiolecta is mediated by DNA repair and MAPK phosphorylation. J Exp Bot. 2012 Sep;63(14):5259-74. PubMed PMID: 22859678. PubMed Central PMCID: PMC3430997.
- Kuwata A, Hama T, Takahashi M. Ecophysiological characterization of two life forms, resting spores and resting cells, of a marine planktonic diatom, Chaetoceros pseudocurvisetus, formed under nutrient depletion. Marine Ecology Progress Series. 1993 Dec 30:245-55.
- Hori T, Norris RE, Chihara M. Studies on the ultrastructure and taxonomy of the genusTetraselmis (Prasinophyceae). Journal of Plant Research. 1982 Mar 1;95(1):49-61.
- Fábregas J, Abalde J, Herrero C, Cabezas B, Veiga M. Growth of the marine microalga Tetraselmis suecica in batch cultures with different salinities and nutrient concentrations. Aquaculture. 1984 Dec 1;42(3-4):207-15.
- Richter PR, Hader D-P, Goncalves RJ, Marcoval MA, Villafane VE, Helbling EW. Vertical migration and motility responses in three marine phytoplankton species exposed to solar radiation. Photochem Photobiol. 2007 Jul-Aug;83(4):810-7. PubMed PMID: 17645651.
- Goes JI, Handa N, Taguchi S, Hama T. Effect of UV-B radiation on the fatty acid composition of the marine phytoplankter Tetraselmis sp.: relationship to cellular pigments. Marine Ecology Progress Series. 1994 Nov 17:259-74.
- Adarme-Vega TC, Thomas-Hall SR, Lim DKY, Schenk PM. Effects of long chain fatty acid synthesis and associated gene expression in microalga Tetraselmis sp. Mar Drugs. 2014 Jun 4;12(6):3381-98. PubMed PMID: 24901700. PubMed Central PMCID: PMC4071582.
- Norris RE. Prasinophytes. In'Developments in Marine Biology. Vol. 2. Phytoflagellates'.(Ed. ER Cox.) pp. 85-145.
- Levine JS, Hays PB, Walker JC. The evolution and variability of atmospheric ozone over geological time. Icarus. 1979 Aug 1;39(2):295-309.
- Montero O, Klisch M, Häder DP, Lubian LM. Comparative sensitivity of seven marine microalgae to cumulative exposure to ultraviolet-B radiation with daily increasing doses. Botanica Marina. 2002 Jul 4;45(4):305-15.
- NOUCHI I. Effects of ultraviolet-B (UV-B) irradiation on growth of cucumber, radish and kidney bean plants. Journal of Agricultural Meteorology. 1991 Mar 10;46(4):205-14.
- Guillard RRL, Ryther JH. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonulaconfervacea (Cleve) Gran. Can J Microbiol. 1962 Apr;8:229-39. PubMed PMID: 13902807.
- Dalzell BJ, Minor EC, Mopper KM. Photodegradation of estuarine dissolved organic matter: a multi-method assessment of DOM transformation. Organic Geochemistry. 2009 Feb 28;40(2):243-57.
- Holm-Hansen O, Lorenzen CJ, Holmes RW, Strickland JD. Fluorometric determination of chlorophyll. ICES Journal of Marine Science. 1965 Dec 1;30(1):3-15.
- Welschmeyer NA. Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnology and Oceanography. 1994 Dec 1;39(8):1985-92.
- Satoh H, Yamaguchi I, Kokubun N, Aruga Y. Application of infrared absorption spectrometry for measuring the photosynthetic production of phytoplankton by the stable 13C isotope method. La Mer.1985; 23:171−176.
- Hama T, Miyazaki T, Ogawa Y, Iwakuma T, Takahashi M, Otsuki A, Ichimura S. Measurement of photosynthetic production of a marine phytoplankton population using a stable 13 C isotope. Marine Biology. 1983 Mar 1;73(1):31-6.
- Helbling EW, Villafane V, Ferrario M, Holm-Hansen O. Impact of natural ultraviolet radiation on rates of photosynthesis and on specific marine phytoplankton species. Marine Ecology Progress Series. 1992 Feb 18:89-100.
- Carreto JJ, Carignan MO.Mycosporine-like amino acids: relevant secondary metabolits. Chemical and ecological aspects. Mar Drugs. 2011 Mar 21;9(3):387-446. PubMed PMID: 21556168. PubMed Central PMCID: PMC3083659.
- Karentz D, Cleaver JE, Mitchell DL. Cell survival characteristics and molecular responses of Antarctic phytoplankton to ultraviolet‐B radiation. Journal of Phycology. 1991 Jun 1;27(3):326-41.
- Buma AG, Boelen P, Jeffrey WH. UVR-induced DNA damage in aquatic organisms. UV effects in aquatic organisms and ecosystems. 2003:291-327.
- Francko DA, Heath RT. UV‐sensitive complex phosphorus: Association with dissolved humic material and iron in a bog lake. Limnology and Oceanography. 1982 May 1;27(3):564-9.
- Osburn CL, Morris DP. Photochemistry of chromophoric dissolved organic matter in natural waters. UV effects in aquatic organisms and ecosystems. 2003;1:185-217.
- Donkor V, Häder DP. Effects of solar and ultraviolet radiation on motility, photomovement and pigmentation in filamentous, gliding cyanobacteria. FEMS Microbiology Letters. 1991 Dec 1;86(2):159-68.
- Behrenfeld MJ, Hardy JT, Lee H. Chronic Effects Of Ultraviolet‐B Radiation On Growth And Cell Volume Of Phaeodactylum Tricornutum (Bacillariophyceae). Journal of Phycology. 1992 Dec 1;28(6):757-60.
- Masi A, Melis A. Morphological and molecular changes in the unicellular green alga Dunaliella salina grown under supplemental UV-B radiation: cell characteristics and Photosystem II damage and repair properties. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 1997 Aug 22;1321(2):183-93.
- Llabrés M, Agustí S. Effects of ultraviolet radiation on growth, cell death and the standing stock of Antarctic phytoplankton. Aquatic Microbial Ecology. 2010 Mar 31;59(2):151-60.
- Laws EA, Taguchi S, Hirata J, Pang L. Continued studies of high algal productivities in a shallow flume. Biomass. 1986 Jan 1;11(1):39-50.
- Dubinsky Z, Falkowski PG, Wyman K. Light harvesting and utilization by phytoplankton. Plant and Cell Physiology. 1986 Oct 1;27(7):1335-49.
- Berner T, Dubinsky Z, Wyman K, Falkowski PG. Photoadaptation and the “package” effect in Dunaliella tertiolecta (Chlorophyceae). Journal of Phycology. 1989 Mar 1;25(1):70-8.
- PréZelin BB, Moline MA, Matlick HA. Ice Colors' 93: Spectral UV radiation effects on Antarctic frazil ice algae. Antarctic sea ice: biological processes, interactions and variability. 1998 Feb 4:45-83.
- Kaneshiro T, Hamasaki K, Toda T, Taguchi S. Effects of ultraviolet radiation(UVB) on the growth of Chaetoceros gracilis. InProceedings of the 14 th international diatom symposium, Tokyo, Japan, September 2- 8 1996. 1999 (pp. 241-248).
- Chisti Y. Biodiesel from microalgae. Biotechnology advances. 2007 Jun 30;25(3):294-306.
- Obata M, Toda T, Taguchi S. Using chlorophyll fluorescence to monitor yields of microalgal production. Journal of applied phycology. 2009 Jun 1;21(3):315-9.
- Malapascua JR, Jerez CG, Sergejevová M, Figueroa FL, Masojídek J. Photosynthesis monitoring to optimize growth of microalgal mass cultures: application of chlorophyll fluorescence techniques. Aquatic Biology. 2014 Nov 20;22:123-40.