Effects Of Tirzepatide On Kidney Function and Outcomes In Patients With Type 2 Diabetes
Nasser Mikhail, MD, MSc
Endocrinology Division, Department of Medicine, Olive-view-UCLA Medical Center, David-Geffen-UCLA School of Medicine, CA, USA
*Corresponding Author
Nasser Mikhail, MD,
Endocrinology Division, Department of Medicine, Olive-view-UCLA Medical Center, David-Geffen-UCLA School of Medicine, CA, USA.
Email Id: nmikhail@dhs.lacounty.gov
Received: October 31, 2022; Accepted: November 15, 2022; Published: November 23, 2022
Citation: Nasser Mikhail. Effects Of Tirzepatide On Kidney Function and Outcomes In Patients With Type 2 Diabetes. Int J Diabetol Vasc Dis Res. 2022;10(03):284-286.
Copyright: Nasser Mikhail©2022. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Recent data suggest that tirzepatide may have kidney protective effects.
Objective: To provide an appraisal of the renal effects of tirzepatide.
Methods: Pubmed search until October 27, 2022. Search terms were tirzepatide, kidneys, albuminuria, diabetes, GIP, GLP-1
agonists. Pertinent clinical trials and reviews were included.
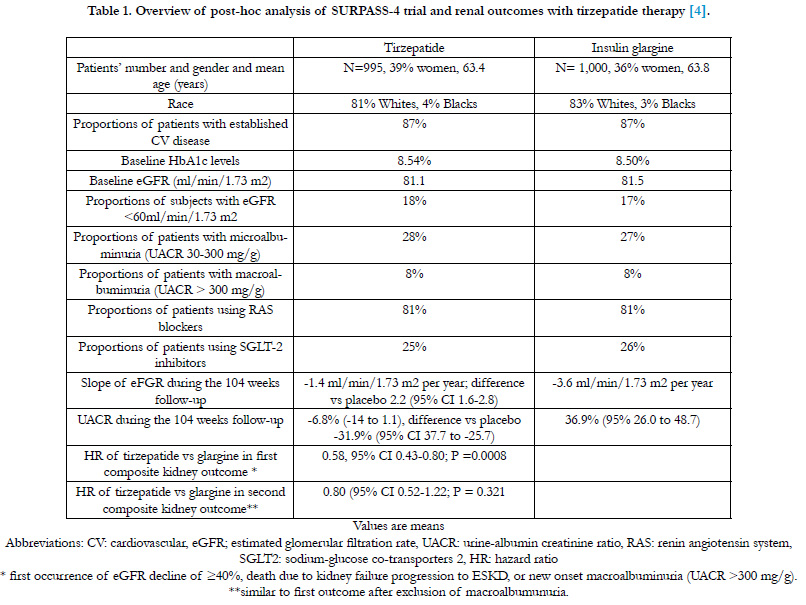
Results: SURPASS-4 trial was a randomized trial comparing efficacy and safety of tirzepatide versus insulin glargine in patients
with type 2 diabetes. A post-hoc analysis of SURPASS-4 compared renal outcomes between the 2 treatment modalities.
This analysis showed that, compared with insulin glargine, tirzepatide slowed progression of kidney disease by decreasing the
decline in estimated glomerular filtration rate (eGFR) During 104 weeks of follow-up, the mean eGFR slope was -1.4 ml/
min/1.73 m2 per year and -3.6 ml/min/1.73 m2 per year in the tirzepatide group and the insulin group, respectively; betweengroup
difference 2.2 (95% CI 1.6-2.8). Tirzepatide effect on eGFR was more pronounced in patients with baseline eGFR <
60 ml/min/1.73 m2 compared with those with eGFR =60 ml/min/1.73 m2. Urine albumin creatinine ratio (UACR) remained
relatively stable in the tirzepatide group but increased progressively in the insulin group. During the 104 weeks of follow-up,
UACR was decreased by 31.9% (95% CI 37.7 to 25.7) with tirzepatide versus insulin glargine. Tirzepatide reduced the risk of
a composite kidney outcome by 42% compared with insulin glargine; hazard ratio (HR) 0.58, 95% CI 0.43-0.80; P =0.0008).
This effect was mainly driven by reduction in new-onset albuminuria. Secondary analyses of trials of glucagon-like peptide-1
(GLP-1) agonists also showed that these agents might have beneficial kidney actions.
Conclusions: Preliminary data suggest that tirzepatide may exert several kidney protective effects in patients with type 2
diabetes. These effects should be examined as primary outcomes in clinical trials including patients with wide spectrum of
renal function at baseline.
2.Philosophy and Discussion
3.Conclusion
4.Acknowledgement
5.References
Keywords
Tirzepatide; type 2 Diabetes; Kidney; Albuminuria; GLP-1 Agonists.
Introduction
Tirzepatide (LY3298176) is a dual receptor agonist of the 2 incretin
hormones: glucose-dependent insulinotropic polypeptide
(GIP) and glucagon-like peptide-1 (GLP-1) that was initially introduced
as anti-diabetic agent [1]. Tirzepatide (Mounjaro) was
approved in May 2022 by the Federal Drug Administration (FDA)
based on a series of phase 3 clinical trials called SURPASS-1
through 5 [1, 2]. SURPASS-4 was an open-label, randomized
multinational trial comparing the efficacy and safety of tirzepatide
with insulin glargine in patients with type 2diabetes and high
cardiovascular (CV) risk [3]. Study results showed superiority of
the 3 doses of tirzepatide (5,10,15 mg once weekly) over insulin
glargine (mean dose at 52 weeks 43.5 units/d) in terms of reduction
in hemoglobin A1c (HbA1c) levels, body weight, systolic
blood pressure (SBP), and frequency of hypoglycemia. However,
tirzepatide was less tolerated than insulin glargine with treatment
discontinuation rates of 9-11% versus 5% with glargine, mainly
due to gastrointestinal adverse effects [3]. Recently [4] performed
a post-hoc analysis of SURPASS-4 trial focusing on kidney effects
of tirzepatide versus insulin glargine over a median duration of
follow-up of 85 weeks. The study had 2 composite kidney outcomes.
The first consisted of time to first occurrence of eGFR
decline of =40%, death due to kidney failure progression to endstage
kidney disease (ESKD), or new onset macroalbuminuria
(UACR >300 mg/g). The second composite kidney outcome was similar to the first but excluded new onset macroalbuminuria [4].
This analysis represents the only available data with respect to the
renal effects of tirzepatide. Overview and main results of this
post-hoc analysis of SURPASS-4 trial are summarized in table 1
and discussed further below.
Effects Of Tirzepatide On Glomerular Filtration Rate
Tirzepatide slowed the rate of worsening of renal function with
time as reflected by the slope of decline of eGFR. Thus, during
the 104 weeks follow-up, the mean eGFR slope was -1.4
ml/min/1.73 m2 per year in the tirzepatide group and -3.6 ml/
min/1.73 m2 per year in the insulin group, between-group difference
2.2 (95% CI 1.6-2.8) [4]. Importantly, tirzepatide effect on
eGFR was more pronounced in patients with baseline eGFR <
60 ml/min/1.73 m2 compared with those with eGFR =60 ml/
min/1.73 m2, i.e participants with worse prevalent kidney function
got more benefit [4]. Meanwhile, the renal protective effects
of tirzepatide were independent of use of other agents known to
slow kidney disease such as angiotensin-converting enzyme inhibitors
(ACEI), angiotensin-receptor blockers (ARB), and sodiumglucose
co-transporter-2 (SGLT2) inhibitors [4]. However, in the
first 12 weeks after randomization, the fall in eGFR was more evident
in the tirzepatide group than in the insulin group [4]. In fact,
this acute initial dip in eGFR was also demonstrated with use of
other renoprotective agents such as ARB, SGLT2 inhibitors, and
the non-steroidal aldosterone antagonist finerenone [5-7]. The exact
cause of this early transient decline in eGFR is unclear but is
believed to be linked to long-term kidney protection [5].
Table 1. Overview of post-hoc analysis of SURPASS-4 trial and renal outcomes with tirzepatide therapy [4].
Effects Of Tirzepatide On Albuminuria
Inspection of the time curves of UACR in the insulin glargine and
tirzepatide groups showed that maximum reduction of UACR
with tirzepatide was observed at 42 weeks (approximately 20%
reduction) followed by gradual rebound to attain a non-significant
reduction of 4.4% after 102 weeks. On the other hand, UACR
progressively increased from baseline in the insulin glargine group
to reach a 56.7% increase at 104 weeks [4]. Overall, during the
104 weeks of follow-up, the between-group difference in UACR
was -31.9% (95% CI -37.7 to -25.7) in favor of tirzepatide [4]. In
addition, tirzepatide decreased the likelihood of progression to
more severe stages of albuminuria [4]. Thus, the HR for worsening
UACR stage for tirzepatide versus insulin was 0.43 (95%
CI 0.27-0.70) [4]. Conversely, patients assigned to tirzepatide had
greater likelihood of regression from microalbuminuria (UACR
30-300 mg/g) to normal UACR or from macroalbuminuria to either
microalbuminuria or normal UACR, HR 1.93 (95% CI 97
1.51-2.57) [4].
Effects Of Tirzepatide On Kidney Outcomes
Compared with insulin glargine, tirzepatide reduced the risk of
the first composite kidney outcome by 42% (HR 0.58, 95% CI
0.43-0.80; P =0.0008) [4]. This effect was mainly driven by reduction in new-onset albuminuria. This conclusion was based on
the fact that the effect of tirzepatide on the second composite
kidney outcome that excluded new onset macroalbuminuria was
no longer significant, HR 0.80 (95% CI 0.52-1.22; P =0.321) [4].
Mechanisms Of Renal Effects Of Tirzepatide
The mechanisms of tirzepatide renal beneficial effects are not fully
understood but are likely multifactorial. The significant decrease
of hemoglobin A1c values, body weight and SBP by tirzepatide
are likely among the reasons [3]. However, the relative contribution
of these factors is unclear because adjustment for changes in
HbA1c levels and weight did not change the results [4]. However,
adjustment for changes in SBP was not reported [4]. A direct effect
of tirzepatide on the kidneys cannot be excluded. That the
renal effects of tirzepatide remained unchanged regardless of use
of ACEI, ARB or SGLT2 inhibitors suggest that the underlying
mechanisms of renoprotection of tirzepatide were different from
those agents [4].
Effects of GLP-1 agonists on kidney function
Several clinical trials evaluated the effects of GLP-1 agonists on
renal function as secondary outcomes [8]. In a meta-analysis of
6 large trials, Sattar et al [8] showed that GLP-1 agonists reduced
kidney outcomes by 21% (HR 0.79; 95% CI 0.73-0.87, P<0.0001).
These outcomes were a composite that consisted of new onset
macroalbuminuria, doubling of serum creatinine or a least a 40%
decline in eGFR, kidney replacement therapy or death due to kidney
disease [8]. Moreover, after excluding the short-acting GLP-
1 agonist lixisenatide, treatment with GLP-1 agonists decreased
worsening kidney function (defined as either doubling of serum
creatinine or =40% decline in eGFR) by 18% (HR 0.82; 95% CI
069-098, P=0.03) [8].
In an exploratory analysis of the REWIND trial (n=9,901, mean
eGFR 76.9 ml/min/1.73 m2) with median follow-up of 4.5 years,
[9] evaluated renal outcomes of the GLP-1 agonist dulaglutide.
These outcomes were a composite of new onset macroalbuminuria,
a sustained decline in eGFR of =30% or renal replacement
therapy [9]. Thus, dulaglutide therapy was associated with reduction
in this composite outcome versus placebo; HR 0.85 (95% CI
0.77-0.93, P=0.0004) [9]. Interestingly, in terms of the individual
components of the renal outcome, the clearest effect of dulaglutide
was the decrease in new onset macroalbuminuria; HR 0.77
(95% CI, 0.68-0.87; P< 0.0001) [9]. Similarly, in the LEADER
trial, Mann et al [10] reported that liraglutide decreased the renal
composite outcome (new onset macroalbuminuria, doubling of
serum creatinine, ESKD, or death due to renal disease) versus placebo,
HR 0.78 (95% CI 0.67-0.92; P=0.003). Again, this decrease
in the renal outcome was driven primarily by the significant reduction
of macroalbuminuria, HR 0.74 (95% 0.60-0.91; P=0.004)
[10]. In a recent analysis of the LEADER trial and SUSTAIN-6
trial of semaglutide [11] showed that the kidney-protective effects
of liraglutide and semaglutide were more pronounced in patients
with pre-existing chronic kidney disease (CKD) having eGFR <
60 ml/min/1.73 m2.
Conclusions And Current Needs
Post-hoc analysis of SURPASS-4 trial suggests for the first time
that tirzepatide may delay progression of diabetic nephropathy
by slowing the decline in eGFR and decreasing albuminuria.
These observations were primarily recorded in patients with type
2 diabetes and relatively preserved kidney function. It is essential
therefore to confirm these results in adequately powered randomized
studies across a wide spectrum of baseline kidney function.
Likewise, secondary analysis of trials of different GLP-1 agonists
suggest that these agents may exert renal protective effects.
The FLOW trial is a large (n=3,508) randomized trial underway
to evaluate the kidney effects of the GLP-1 agonist semaglutide
versus placebo in patients with type 2 diabetes and CKD [11]. The
primary outcome in FLOW is the composite of kidney failure,
a persistent =50% reduction in eGFR, and kidney or CV death
[11]. Confirmation of renal protection by tirzepatide and GLP-
1 agonists will add constitute another advantage of these agents
in addition to their established efficacy in glycemic control and
weight loss.
References
- Mikhail N. Who are the best candidate patients with diabetes for tirzepatide?. Austin Diabetes Res. 2022;7(1):1-5.
- Tirzepatide (Mounjaro). Prescribing information. Eli Elly and Company, Indianapolis, IN 46285, 2022, USA.
- Del Prato S, Kahn SE, Pavo I, Weerakkody GJ, Yang Z, Doupis J, et al; SURPASS-4 Investigators. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. 2021 Nov 13;398(10313):1811-1824. PubMed PMID: 34672967.
- Heerspink HJL, Sattar N, Pavo I, Haupt A, Duffin KL, Yang Z, et al. Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: post-hoc analysis of an open-label, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2022 Nov;10(11):774-785. Pub- Med PMID: 36152639.
- Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJ, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011 Aug;80(3):282-7. PubMed PMID: 21451458.
- Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al; CREDENCE Trial Investigators. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019 Jun 13;380(24):2295-2306. PubMed PMID: 30990260.
- Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al; FIDELIO- DKD Investigators. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N Engl J Med. 2020 Dec 3;383(23):2219- 2229. PubMed PMID: 33264825.
- Sattar N, Lee MMY, Kristensen SL, Branch KRH, Del Prato S, Khurmi NS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021 Oct;9(10):653- 662. PubMed PMID: 34425083.
- Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al; REWIND Investigators. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebocontrolled trial. Lancet. 2019 Jul 13;394(10193):131-138. PubMed PMID: 31189509.
- Mann JFE, ōrsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, et al; LEADER Steering Committee and Investigators. Liraglutide and Renal Outcomes in Type 2 Diabetes. N Engl J Med. 2017 Aug 31;377(9):839-848. PubMed PMID: 28854085.
- Shaman AM, Bain SC, Bakris GL, Buse JB, Idorn T, Mahaffey KW, et al. Effect of the Glucagon-Like Peptide-1 Receptor Agonists Semaglutide and Liraglutide on Kidney Outcomes in Patients With Type 2 Diabetes: Pooled Analysis of SUSTAIN 6 and LEADER. Circulation. 2022 Feb 22;145(8):575-585. PubMed PMID: 34903039.