Role of SGLT-2 Inhibitors in Treatment of Patients with Type 2 Diabetes and NAFLD
Nasser Mikhail, MD*
Endocrinology Division, Department of Medicine, Olive-view-UCLA Medical Center, David-Geffen-UCLA School of Medicine, CA, USA
*Corresponding Author
Nasser Mikhail, MD,
Endocrinology Division, Department of Medicine, Olive-view-UCLA Medical Center, David-Geffen-UCLA School of Medicine, CA, USA.
Email Id: nmikhail@dhs.lacounty.gov
Received: February 21, 2022; Accepted: March 10, 2022; Published: March 11, 2022
Citation: Nasser Mikhail. Role of SGLT-2 Inhibitors in Treatment of Patients with Type 2 Diabetes and NAFLD. Int J Diabetol Vasc Dis Res. 2022;10(02):279-283. doi: dx.doi.org/10.19070/2328-353X-2100053
Copyright: Nasser Mikhail©2022. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: The role of sodium-glucose cotransporters-2 (SGLT-2) inhibitors in treatment of non-alcoholic fatty liver
disease (NAFLD) is unclear.
Objective: To examine the possible therapeutic role of SGLT-2 inhibitors in patients with type 2 diabetes and NAFLD.
Methods: Pubmed search of English literature up to February 17, 2022. Search terms are fatty liver, SGLT-2, sodium-glucose
cotransporter-2 inhibitors, diabetes, weight loss, liver biopsy. Randomized studies are reviewed with focus placed on doubleblind,
placebo-controlled trials. Meta-analyses and pertinent reviews are also included.
Results: Six randomized double-blind placebo-controlled trials examined the effects of SGLT-2 inhibitors on intra-hepatic
fat content (IHFC) over 8-24 weeks mostly by magnetic resonance imaging (MRI) techniques. Two trials reported significant
reduction in IHFC with SGLT-2 inhibitors, one reported borderline statistically significant reduction, and the remaining 3
trials showed a trend towards amelioration of IHFC that did not reach statistical significance. One placebo-controlled study
showed that empagliflozin (10 mg/d) may decrease IHFC in patients with NAFLD without diabetes. Weight loss induced by
SGLT-2 inhibitors appears to be a major factor in decreasing IHFC. Two small non-randomized biopsy studies showed that
canagliflozin could improve histopathologic disease severity in patients with confirmed NAFLD and type 2 diabetes.
Conclusions: SGLT-2 inhibitors are promising agents for treatment of NAFLD. Long-term randomized studies using paired
hepatic biopsies as endpoints are urgently needed to establish the therapeutic role of SGLT-2 inhibitors in NAFLD in patients
with and without diabetes.
2.Philosophy and Discussion
3.Conclusion
4.Acknowledgement
5.References
Keywords
Fatty Liver; Sodium-Glucose Cotransporter-2 Inhibitors; Diabetes; Liver Biopsy.
Abbreviations
HA1c: Hemoglobin A1c, MRI: Magnetic Resonance Imaging, GGT; Gamma-Glutamyl Transferase, ALT:
Alanine Aminotransferase, AST: Aspartate Aminotransferase.
Introduction
There are multiple reasons to evaluate SGLT-2 inhibitors for
treatment of NAFLD. First, the prevalence of NAFLD in patients
with type 2 diabetes is very common ranging from 29.6%
to 87.1%, with an estimated pooled prevalence of 59.6% (95%
CI; 54.3–64.9%) [1]. Second, NAFLD is associated with increased
incidence of cardiovascular and chronic kidney disease [2, 3]. In
the meantime, there is strong evidence from randomized trials
that SGLT-2 inhibitors may reduce cardiorenal events in patients
with type 2 diabetes [4, 5]. Third, uncontrolled glycemic control
may be linked to increased severity of histological changes of
NAFLD [6]. Thus, every 1% increase in mean hemoglobin A1c
(HbA1c) levels was associated with 15% higher odds of increased
fibrosis stage (odds ratio 1.15; 95% CI, 1.01-3.01) [6]. Therefore,
it is possible that improving glycemic status by SGLT-2 inhibitors
could decrease severity and progression of NAFLD. Fourth,
while weight loss is the cornerstone therapy for NAFLD, the use
of SGLT-2 inhibitors results in mild weight loss of approximately
2-3 kg [4, 5]. This magnitude of weight loss, although modest,
could contribute to amelioration of NAFLD [7]. Finally, there is
currently no medications approved by the Federal Drug Administration
(FDA) for treatment of NAFLD. Hence, it is necessary
to pursue research efforts to find effective and safe drugs to fill
this large gap. The main purpose of this article is to summarize
human data regarding the potential therapeutic benefits of SGLT-2 inhibitors in NAFLD. Special emphasis will be placed on the
strongest evidence derived from randomized, double-blind-placebo
controlled trials.
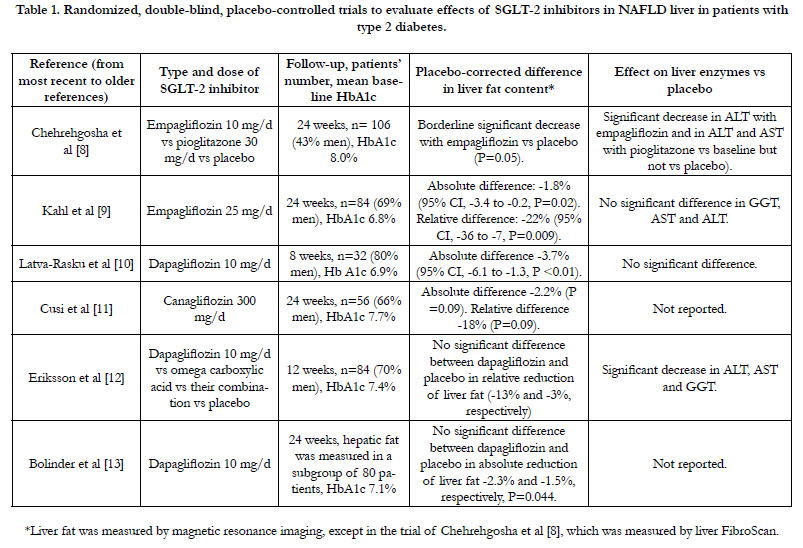
Randomized, Double-Blind, Placebo-Controlled Studies
Six randomized double-blind, placebo-controlled trials (summarized
in table 1) are available for evaluation of effects of various
SGLT-2 inhibitors on IHFC [8-13]. Intrahepatic fat was measured
by MRI in 5 studies, and by transient hepatic elastography (liver
FibroScan) in one study [8] (table 1). The studies were generally
small (32-106 patients) and of short duration (8-24 weeks). In 5
studies, majority of patients (56-70%) were men (table 1). NAFLD
was not a prerequisite inclusion criterion, except in the trial conducted
by Eriksson et al [12] in which all patients had NAFLD at
baseline (defined as proton density fat fraction of =5.5% by MRI)
and in the trial by Chehrehgosha et al [8] (defined as controlled attenuation
parameter =238 dB/m in transient hepatic elastography
by FibroScan). In 2 of the 6 studies, dapagliflozin and empagliflozin
were associated with significant decrease in IHFC after 8
weeks and 24 weeks, respectively compared with placebo (table 1)
[9, 10]. In one trial [8], empagliflozin 10 mg/d was associated with
borderline significant reduction in IHFC versus placebo (P=0.05).
In the remaining 3 studies using canagliflozin and dapagliflozin,
there was a trend toward reduction in IHFC versus placebo that
did not attain statistical significance [11-13] (table 1). Overall, the
magnitude of reduction in IHFC was modest [9, 10] (table 1). For
instance, placebo-corrected relative reduction in IHFC of 22%
was demonstrated with empagliflozin [9]. Meanwhile, emerging
data suggests that a relative reduction of at least 30% of IHFC
may be clinically meaningful [14]. Of note, all 6 studies included
overweight and obese patients with fairly controlled type 2 diabetes
(mean baseline HbA1c 6.8-7.7%). However, in one open-label,
and non-placebo-controlled trial, Kuchay et al [15] included patients
with poorly controlled type 2 diabetes (mean HbA1c 9.0%)
and all subjects had documented hepatic steatosis at baseline (defined
as liver fat > 6% as measured by MRI-proton density fraction).
After 20 weeks, small dose of empagliflozin (10 mg/d) was
associated with significantly greater relative reduction of IHFC
of 4% than the control group receiving standard diabetes care
(P<0.0001) [15].
Table 1. Randomized, double-blind, placebo-controlled trials to evaluate effects of SGLT-2 inhibitors in NAFLD liver in patients with type 2 diabetes.
Comparison Head To Head Trials
Few randomized trials compared the effect of SGLT-2 inhibitors
with other agents on fatty liver in patients with type 2 diabetes.
The placebo-controlled trial of Chehrehgosha et al [8] mentioned
above included a third group of patients randomized to pioglitazone
(30 mg/d), another anti-diabetic agent that showed previous
efficacy in decreasing IHFC in patients with and without diabetes
[16, 17]. After 24 weeks, the decrease in liver fibrosis (evaluated by
liver stiffness measurement) was significantly greater with empagliflozin
compared with pioglitazone [8]. In one open-label Japanese
study, Kinoshita et al [18] compared effects of dapagliflozin
(5 mg/d), pioglitazone (mean daily dose 17.3 mg), and glimepiride
(mean daily dose 0.9 mg) on NAFLD assessed by change in liverto-
spleen (L/S) ratio on abdominal computed tomography. All
included patients (n=98) had NAFLD at baseline defined as L/S
ratio <1.0, After 28 weeks, all 3 groups of patients had similar
glycemic control. Yet, glimepiride had no impact on L/S ratio,
whereas dapagliflozin and pioglitazone had similar effects on decreasing IHFC as reflected by a significant increase in L/S ratio
[18]. Using the same previous methodology to evaluate NAFLD,
Ito et al [19] compared the effects of ipragliflozin (50 mg/d) and
pioglitazone (15-30 mg/d) on NAFLD in an open-label study including
66 patients with poorly controlled type 2 diabetes (mean
baseline HbA1c 8.3%). After 24 weeks, the effects of ipragliflozin
and pioglitazone on L/S ratio was similar [19]. Taken together,
the above head to head trials suggest that SGLT-2 inhibitors are
at least equivalent to pioglitazone in reducing IHFC. Interestingly,
in the 3 previous studies, pioglitazone therapy was associated with
weight gain, whereas the SGLT2 inhibitors (empagliflozin, dapagliflozin
and ipragliflozin) were associated with weight loss [8, 18,
19]. This finding suggests that different mechanisms of actions
may be involved in the decrease of IHFC between SGLT-2 inhibitors
and pioglitazone. In support of this notion, is the demonstration
of an “additive effect” on reduction of IHFC when
both agents are used concomitantly as outlined in the coming
paragraph.
Combination Therapy
In a small (n=44) randomized trial of 26 week-duration, Han et al
[20] showed that addition of ipragliflozin (50 mg/d) to ongoing
pioglitazone therapy further decreased IHFC assessed by transient
liver elastography. In a post-hoc analysis of a randomized
trial (DURATION-8), Gastaldelli et al [21] compared efficacy of
dapagliflozin 10 mg/d, the GLP-1 agonist exenatide (2 mg once
weekly by subcutaneous injection), or their combination in reducing
severity of NAFLD. After 52 weeks, they found that combination
therapy was superior to each treatment alone in ameliorating
serum markers of hepatic steatosis and fibrosis [21].
Histological studies
Unfortunately, data from histological studies are very limited.
Akuta et al [22] examined the effects of canagliflozin 100 mg
bid in 5 patients with type 2 diabetes and histological evidence
of NAFLD (defined as steatosis in 5% or more of hepatocytes).
After 24 weeks, repeated liver biopsies showed improvement in
the NAFLD activity score, which represents the sum of steatosis,
lobular inflammation, and hepatocyte ballooning scores in all 5
patients [22]. In another pilot study, Lai et al [23] evaluated empagliflozin
25 mg/d in 9 patients with type 2 diabetes and confirmed
NAFLD. These authors used historical placebo group for
comparison. After 24 weeks, all histological histologic outcomes
(steatosis, fibrosis, ballooning), either remained unchanged or improved,
except in one patient who had worsening ballooning [23].
Thus, compared with historical placebo, empagliflozin therapy
was associated with significantly greater improvements in steatosis
(67% vs 26%, P=0.025), ballooning (78% vs 34%, P =0.024),
and fibrosis (44% vs 6%, P=0.008) [23]. These preliminary data
are encouraging. Clearly, they require confirmation by randomized
double-blind and placebo-controlled histological studies.
Effects of SGLT-2 inhibitors on NAFLD in patients without
diabetes
In one randomized double-blind and placebo controlled, Taheri
et al [24] evaluated effects of small dose empagliflozin (10 mg/d)
on the degree of steatosis in 90 patients without diabetes by liver
FibroScan. After 24 weeks, the percentage of patients of patients
with improved steatosis was significantly greater in the empagliflozin
group than the placebo group, 37.2% and 17%, respectively
(P=0.035) [24]. Of note, this significant difference was observed
only in the subgroup of patients (n=44) with significant steatosis
at baseline [24]. Furthermore, fibrosis score was significantly improved
in the empagliflozin group vs placebo (P==0.039) [24].
Effects of SGLT-2 inhibitors on transaminases
Elevation of serum aminotransaminases is considered a surrogate
marker of NAFLD. However, alanine aminotransferase (ALT)
and aspartate aminotransferase (AST) can be normal in many cases
of NAFLD including patients with advanced fibrosis [25]. As
seen in table 1, effects of SGLT-2 inhibitors on transaminases are
variable, with no significant effect in most studies. A meta-analysis
of randomized trials showed that treatment with SGLT-2 inhibitors
significantly decreased levels of ALT (9 trials), and gammaglutamyl
transferase (GGT) (6 trials), but not AST (9 trials) [3].
Predictors to favorable response to SGLT-2 inhibitors
Preliminary data from placebo-controlled trials suggest that the
following 3 factors may predict a favorable response to SGLT-2
inhibitors in terms of reduction in IHFC. First, the magnitude of
weight loss as shown in most studies [9, 11]. Second, the decrease
in hepatic fat might be more pronounced in patients with baseline
NAFLD [11, 24]. Third, response to SGLT-2 inhibitors with
respect to gender was only analyzed in the study by Kahl et al [9].
In that study, there was only significant decrease in IHFC among
males [-31% (95% -44 to -14; P=0.002)], but not females [-1%
(95% CI -28 to +37); P=0.96] [9].
Possible mechanisms of reduction of hepatic fat by SGLT-2
inhibitors
The exact mechanisms whereby SGLT-2 inhibitors may decrease
IHFC are not fully elucidated. Possible mechanisms are discussed
below.
Weight loss: While the use of SGLT-2 inhibitors is known to
induce a weight low of approximately 2.5 kg [9, 11, 13], this minor
degree of weight loss appears to be a major factor in decreasing
liver fat. For example, in the study of Kahl et al [9], the difference
in liver fat between the empagliflozin group and placebo group
was no longer statistically significant after adjustment for change
in body weight. Moreover, in the study of Cusi et al [11], significant
correlation between weight loss and decrease in IHFC (correlation
coefficient r =0.58, P<0.001) was observed in patients
randomized to canagliflozin as well as those randomized to placebo.
Conversely, Kuchay et al [15] did not find significant correlation
between weight reduction and liver fat reduction (r=0.218,
P=0.329). Thus, it is possible that factors other than weight loss
could be also involved.
Effect on insulin resistance: Insulin resistance plays a major
role in the pathogenesis of both type 2 diabetes and NAFLD [26].
Meanwhile, there is no evidence that SGLT-2 inhibitors improve
insulin sensitivity either in the whole body [8] or in various tissues
such as the liver, adipose tissue and muscle [9]. In addition, Cusi et
al [11] did not find any effect of canagliflozin on insulin resistance
in muscle and adipose tissue. Although these authors reported
improvement of insulin sensitivity at the level of hepatic tissue by canagliflozin, the drug had no significant effect on IHFC [11].
Glycemic control: The design of 2 trials allowed testing the hypothesis
whether amelioration of glycemic status by SGLT-2 inhibitors
may have a role in decreasing IHFC. Thus, in these 2 studies,
the authors maintained similar HbA1c values throughout the
trials both in patients with controlled type 2 diabetes in the study
of Kahl et al [9], and patients with uncontrolled type 2 diabetes in
the study of Kuchay et al [15]. In both studies, SGLT-2 inhibitors
decreased IHFC despite similar glycemic control between patient
groups. Another finding that supports the concept that reduction
in IHFC by SGLT-2 inhibitors occurs independently of glycemic
control is the observation showing that empagliflozin may
decrease steatosis and fibrosis in patients without diabetes [24].
Other mechanisms: Other potential mechanisms include decrease
in insulin levels by SGLT-2 inhibitors leading to reduction
in hepatic de novo lipid synthesis and increase in glucagon levels.
The latter leads to stimulation of hepatic ß-oxidation of fatty acids
[26, 27]. Further investigations are required to study the relative
contribution of these mechanisms to the decrease in IHFC
by SGLT-2 inhibitors.
Conclusions And Future Needs
Preliminary data suggest that SGLT-2 inhibitors may have beneficial
effects for treatment of NAFLD. Most placebo-controlled
studies relied on MRI-imaging modalities to assess changes of
IHFC after drug intervention. This methodology suffers from
important limitations. Indeed, by analysis of data from 121 patients
with paired liver biopsies and MRI, Bril et al [28] concluded
that quantification of liver fat with MRI techniques may be misleading
as a surrogate marker of treatment response and may not
accurately predict histological improvement of steatohepatitis
after treatment. In fact, liver biopsy remains the gold standard
method to confirm diagnosis of NAFLD and evaluate its response
to different therapeutic interventions. In that respect, the
study of Dapagliflozin Efficacy and Action in NASH (DEAN)
(NCT03723252) is an ongoing phase 3, randomized, placebocontrolled
trial that will recruit 100 biopsy-proven NASH patients
with type 2 diabetes. The primary outcome of the DEAN
trial is to examine the effect of dapagliflozin (10 mg/d) on hepatic
histological lesions after 12 months compared with placebo.
Combat T2 NASH is another randomized 48 week- trial
that compares empagliflozin with the once-weekly glucagon-like
peptide-1 (GLP-1) agonist semaglutide and placebo on histological
liver changes in patients with type 2 diabetes. Since SGLT-2
inhibitors were used with success in patients without diabetes for
cardiac protection [4], it is worth while to extend biopsy studies
to patients with NAFLD without diabetes. Results of these trials
should determine with more certainty the role of SGLT-2 inhibitors
for treatment of NAFLD in general.
References
- Dai W, Ye L, Liu A, Wen SW, Deng J, Wu X, et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: A meta-analysis. Medicine (Baltimore). 2017 Sep;96(39):e8179. PubMed PMID: 28953675.
- Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017 Jun;66(6):1138-1153. PubMed PMID: 28314735.
- Mantovani A, Petracca G, Csermely A, Beatrice G, Targher G. Sodium-Glucose Cotransporter-2 Inhibitors for Treatment of Nonalcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials. Metabolites. 2020 Dec 30;11(1):22. PubMed PMID: 33396949.
- Mikhail N. Sodium glucose co-transporters 2 inhibitors for prevention and treatment of heart failure. J Endocrinology Thyroid Res. 2019;5(2):555-656.
- Mikhail N. SGLT2 inhibitors for the treatment of diabetic nephropathy. Curr Res Diabetes & Obes J. 2019;12(3):555839.
- Alexopoulos AS, Crowley MJ, Wang Y, Moylan CA, Guy CD, Henao R, et al. Glycemic Control Predicts Severity of Hepatocyte Ballooning and Hepatic Fibrosis in Nonalcoholic Fatty Liver Disease. Hepatology. 2021 Sep;74(3):1220-1233. PubMed PMID: 33724511.
- Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017 Oct;67(4):829-846. PubMed PMID: 28545937.
- Chehrehgosha H, Sohrabi MR, Ismail-Beigi F, Malek M, Reza Babaei M, et al. Empagliflozin Improves Liver Steatosis and Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Diabetes Ther. 2021 Mar;12(3):843-861. PubMed PMID: 33586120.
- Kahl S, Gancheva S, Straßburger K, Herder C, Machann J, Katsuyama H, et al. Empagliflozin Effectively Lowers Liver Fat Content in Well-Controlled Type 2 Diabetes: A Randomized, Double-Blind, Phase 4, Placebo-Controlled Trial. Diabetes Care. 2020 Feb;43(2):298-305. PubMed PMID: 31540903.
- Latva-Rasku A, Honka MJ, Kullberg J, Mononen N, Lehtimäki T, Saltevo J, et al. The SGLT2 Inhibitor Dapagliflozin Reduces Liver Fat but Does Not Affect Tissue Insulin Sensitivity: A Randomized, Double-Blind, Placebo- Controlled Study With 8-Week Treatment in Type 2 Diabetes Patients. Diabetes Care. 2019 May;42(5):931-937. PubMed PMID: 30885955.
- Cusi K, Bril F, Barb D, Polidori D, Sha S, Ghosh A, et al. Effect of canagliflozin treatment on hepatic triglyceride content and glucose metabolism in patients with type 2 diabetes. Diabetes Obes Metab. 2019 Apr;21(4):812- 821. PubMed PMID: 30447037.
- Eriksson JW, Lundkvist P, Jansson PA, Johansson L, Kvarnström M, Moris L, et al. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia. 2018 Sep;61(9):1923-1934. Pub- Med PMID: 29971527.
- Bolinder J, Ljunggren Ö, Kullberg J, Johansson L, Wilding J, Langkilde AM, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012 Mar;97(3):1020-31. PubMed PMID: 22238392.
- Caussy C, Reeder SB, Sirlin CB, Loomba R. Noninvasive, Quantitative Assessment of Liver Fat by MRI-PDFF as an Endpoint in NASH Trials. Hepatology. 2018 Aug;68(2):763-772. PubMed PMID: 29356032.
- Kuchay MS, Krishan S, Mishra SK, Farooqui KJ, Singh MK, Wasir JS, et al, Mithal A. Effect of Empagliflozin on Liver Fat in Patients With Type 2 Diabetes and Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial (E-LIFT Trial). Diabetes Care. 2018 Aug;41(8):1801-1808. PubMed PMID: 29895557.
- Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006 Nov 30;355(22):2297-307. PubMed PMID: 17135584.
- Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010 May 6;362(18):1675-85. PubMed PMID: 20427778.
- Kinoshita T, Shimoda M, Nakashima K, Fushimi Y, Hirata Y, Tanabe A, et al. Comparison of the effects of three kinds of glucose-lowering drugs on non-alcoholic fatty liver disease in patients with type 2 diabetes: A randomized, open-label, three-arm, active control study. J Diabetes Investig. 2020 Nov;11(6):1612-1622. PubMed PMID: 32329963.
- Ito D, Shimizu S, Inoue K, Saito D, Yanagisawa M, Inukai K, et al. Comparison of Ipragliflozin and Pioglitazone Effects on Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes: A Randomized, 24-Week, Open- Label, Active-Controlled Trial. Diabetes Care. 2017 Oct;40(10):1364-1372. PubMed PMID: 28751548.
- Han E, Lee YH, Lee BW, Kang ES, Cha BS. Ipragliflozin Additively Ameliorates Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Controlled with Metformin and Pioglitazone: A 24-Week Randomized Controlled Trial. J Clin Med. 2020 Jan 18;9(1):259. PubMed PMID: 31963648.
- Gastaldelli A, Repetto E, Guja C, Hardy E, Han J, Jabbour SA, et al. Exenatide and dapagliflozin combination improves markers of liver steatosis and fibrosis in patients with type 2 diabetes. Diabetes Obes Metab. 2020 Mar;22(3):393-403. PubMed PMID: 31692226.
- Akuta N, Watanabe C, Kawamura Y, Arase Y, Saitoh S, Fujiyama S, et al. Effects of a sodium-glucose cotransporter 2 inhibitor in nonalcoholic fatty liver disease complicated by diabetes mellitus: Preliminary prospective study based on serial liver biopsies. Hepatol Commun. 2017 Feb 27;1(1):46-52. PubMed PMID: 29404432.
- Lai LL, Vethakkan SR, Nik Mustapha NR, Mahadeva S, Chan WK. Empagliflozin for the Treatment of Nonalcoholic Steatohepatitis in Patients with Type 2 Diabetes Mellitus. Dig Dis Sci. 2020 Feb;65(2):623-631. PubMed PMID: 30684076.
- Taheri H, Malek M, Ismail-Beigi F, Zamani F, Sohrabi M, Reza Babaei M, et al. Effect of Empagliflozin on Liver Steatosis and Fibrosis in Patients With Non-Alcoholic Fatty Liver Disease Without Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Adv Ther. 2020 Nov;37(11):4697- 4708. PubMed PMID: 32975679.
- Gawrieh S, Wilson LA, Cummings OW, Clark JM, Loomba R, Hameed B, et al. Histologic Findings of Advanced Fibrosis and Cirrhosis in Patients With Nonalcoholic Fatty Liver Disease Who Have Normal Aminotransferase Levels. Am J Gastroenterol. 2019 Oct;114(10):1626-1635. PubMed PMID: 31517638.
- Gharaibeh NE, Rahhal MN, Rahimi L, Ismail-Beigi F. SGLT-2 inhibitors as promising therapeutics for non-alcoholic fatty liver disease: pathophysiology, clinical outcomes, and future directions. Diabetes Metab Syndr Obes. 2019 Jul 3;12:1001-1012. PubMed PMID: 31308716.
- Daniele G, Xiong J, Solis-Herrera C, Merovci A, Eldor R, Tripathy D, et al. Dapagliflozin Enhances Fat Oxidation and Ketone Production in Patients With Type 2 Diabetes. Diabetes Care. 2016 Nov;39(11):2036-2041. Pub- Med PMID: 27561923.
- Bril F, McPhaul MJ, Caulfield MP, Clark VC, Soldevilla-Pico C, Firpi- Morell RJ, et al. Performance of Plasma Biomarkers and Diagnostic Panels for Nonalcoholic Steatohepatitis and Advanced Fibrosis in Patients With Type 2 Diabetes. Diabetes Care. 2020 Feb;43(2):290-297. PubMed PMID: 31604692.