Estimating the Lag Between Onset and Diagnosis of Diabetes from the Prevalence of Diabetic Retinopathy Among Indian Population
Bansal D1*, Boya CS2, Gudala K2, Rambabu V3, Bhansali A4
1 Assistant Professor, Clinical Research Unit, Department of Pharmacy Practice, National Institute of Pharmaceutical Education and Research, SAS
Nagar, Punjab, India.
2 PhD Scholar, Clinical Research Unit, Department of Pharmacy Practice, National Institute of Pharmaceutical Education and Research, SAS Nagar,
Punjab, India.
3 M.Pharm Student, Clinical Research Unit, Department of Pharmacy Practice, National Institute of Pharmaceutical Education and Research, SAS
Nagar, Punjab, India.
4 Professor, Department of Endocrinology, Post Graduate Institute of Medical Education and Research, Chandigarh, India.
*Corresponding Author
Dipika Bansal,
Assistant Professor, Clinical Research Unit,
Department of Pharmacy Practice,
National Institute of Pharmaceutical Education and Research, SAS Nagar, Punjab, 160062, India.
E-mail: dipikabansal079@gmail.com
Received: November 01, 2016; Accepted: March 09, 2017; Published: March 14, 2017
Citation: Bansal D, Boya CS,Gudala K, Rambabu V, Bhansali A (2017) Estimating the Lag Between Onset and Diagnosis of Diabetes from the Prevalence of Diabetic Retinopathy Among Indian Population. Int J Diabetol Vasc Dis Res,. 5(2), 189-195. doi: dx.doi.org/10.19070/2328-353X-1700039
Copyright: Bansal D© 2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background & Objectives: Type 2 Diabetes Mellitus (T2DM) is often characterized by an asymptomatic phase of around 4-7 years between the onset of diabetes and its clinical diagnosis. Diabetic retinopathy is the one to be observed as an early sign of microvascular complications. The objective of the present study was investigate the time lag between the onset of hyperglycemia and clinical diagnosis of T2DM.
Methods: The present cross-sectional study was done at an outpatient setting of an endocrinology clinic. According to inclusion criteria, consecutive patients subjects of either sex with T2DM either newly diagnosed at the time of first encounter with study investigator or previously diagnosed with any duration of T2DM were eligible to be recruited in the present study. A weighted linear regression analysis was performed to estimate the prevalence of retinopathy at each time point. The period at which the prevalence of retinopathy was zero is back extrapolated from the graph to get onset of diagnosis.
Results and Interpretations: A total of 1407 patients with T2DM are included in present analysis. 52% (n = 725) were females and mean (SD) age was 54.3 (10.1) years. The prevalence of diabetic retinopathy was found to be 13.7% (n = 194) at the time of clinical diagnoses. Prevalence of retinopathy increased linearly with duration of T2DM. Estimated the actual onset of time of diabetes was found to be 11.4 (95% CI, 9.5-15) years before the patients were clinically diagnosed with T2DM.
Conclusions: High diagnostic lag was observed in present study. It indicates that there is a need for continues education, counseling of high risk population on diabetes mellitus and its complications to increase awareness of diabetes and reduction of diagnostic lag.
2.Introduction
3.Methodology
3.1 Study Design and Setting
3.2 Eligibility Criteria
3.3 Variables and Data Sources
3.4 Assessment of Microvascular Complications
3.5 Statistical Analysis
4.Results
4.1 Prevalence of Retinopathy
4.2 Predictors of Retinopathy
5.Discussion
6.Strengths and Limitations
7.Conclusion
8.Acknowledgements
9.References
Keywords
Type 2 Diabetes Mellitus; Diagnostic Lag; Diabetic Retinopathy; Prevalence; Weight Least Square Regression.
Introduction
International diabetic federation 2016 reported that 415 million people are diagnosed with diabetes mellitus (DM) globally having prevalence of 8.8% with nearly half of them remaining undiagnosed at any point of time [1, 2]. Majority of undiagnosed subjects belong to type 2 diabetes mellitus (T2DM). According to Diabetes Atlas 2015, India is having 69.18 million subjects with DM accounting for 14.8 % of global burden [1].
T2DM is a chronic condition characterized by hyperglycemia resulting from a progressive insulin secretary defect in the background of insulin resistance [3]. It is also associated with a prolonged asymptomatic phase of 4-8 years between the onset of diabetic hyperglycemia and clinical diagnosis [4-6]. Delayed clinical diagnoses lead to the development of various micro and macro vascular complications. Diabetic retinopathy (DR) is the most commonly observed earliest sign of microvascular complications [7-10]. Development of DR is a leading cause of blindness in DM subjects. World health organization has attributed 4.8% cases of blindness to DR [11]. The prevalence of DR in India is reported to be in the range of 4.8-53.3% [12-16]. Our research group has reported high prevalence of microvascular complications in newly as well as established T2DM patients in north India [9, 10]. Evidence has also correlated the rising incidence of DR with increasing duration of DM [17].
Progression of DR is directly related to the duration and degree of hyperglycemia [15]. Within a decade of clinical diagnosis, about 80% patients develop DR. This prevalence rises to 90% after 15-20 years of diagnosis and reaches to almost 100% after 20 years of diagnosis [16].
Researchers have also tried to investigate the actual onset of DR before the clinical diagnosis is made. Assuming the correlation between DR and DM duration to be linear, researchers have back extrapolated the regression line of time of clinical diagnosis of DM and prevalence of DR and estimated that the onset of retinopathy could have occurred between 4-9 years prior to clinical diagnosis of DM [5, 18]. The present analysis is performed to investigate the time lag between the onset of hyperglycemia and clinical diagnosis of T2DM in Indian patients. To investigate this, we observed the prevalence of DR with increasing duration of DM (as it is considered to be first micro vascular complication to arise) in a study cohort of patients with T2DM from north Indian population.
Data presented in this manuscript is part of a large prospective study where patients were screened to assess the prevalence and predictors of cancer in patients with T2DM. The cross-sectional study was carried out in an outpatient endocrinology clinic of a public tertiary care hospital, Post Graduate Institute of Medical Education and Research (PGIMER) in north India. The study was initiated after approval by the Institute Ethical Review Committee, PGIMER Chandigarh, India. STROBE (Strengthening the Reporting of Observational studies in Epidemiology) guidelines were followed while reporting the study.
Sample selection: Consecutive eligible patients meeting the inclusion criteria were recruited during July 2011 and June 2013. According to inclusion criteria, adult subjects of either sex with T2DM either newly (<6 months) diagnosed as per American Diabetes Association (ADA) guidelines (random blood sugar (RBS) >200 mg/dL or fasting blood sugar (FBS) >126 mg/ dL or HbA1c ≥ 6.5) at the time of first encounter with study investigator or previously diagnosed with any duration of T2DM were recruited in the original study. The subjects included in the present analysis were further classified according to the duration of their disease. Patients having type 1 diabetes, gestational diabetes and having diabetes for more than 20 years were excluded from the study.
Anthropometric measurements including weight, height (using stadiometer), body mass index (BMI; kg/m2), and waist circumference (using inelastic and flexible tape at the midpoint between the lower margin of the least palpable rib and top of the iliac crest nearest to 0.1 cm) were done at the time of recruitment.
Information about lifestyle characteristics like smoking and alcohol consumption were obtained through patient interview at the time of recruitment. Clinical systolic blood pressure (SBP) and diastolic blood pressure (DBP) levels, serum lipids, blood glucose and glycated hemoglobin (HbA1c), and hepatic and renal function levels were extracted from the available clinical records. Blood pressure was measured in the sitting position in right arm with a mercury sphygmomanometer (Diamond Deluxe BP apparatus, BP Instruments, Pune, India), and the participants were considered to be hypertensive if they were taking antihypertensive medication (as documented in clinic records) or SBP ≥ 140 mmHg or DBP ≥ 90 mmHg. HbA1c was measured using the variant machine (Bio-Rad Laboratories, Hercules, CA, USA). Serum cholesterol (cholesterol esterase oxidase-peroxidase amidopyrine method), serum triglycerides (glycerol phosphate oxidase-peroxidaseamidopyrine method), and high density lipoprotein cholesterol (direct method polyethylene glycol-pretreated enzymes) were measured using the Beckman Coulter AU 2700/480 Autoanalyser (Beckman AU (Olympus), Ireland).
All study participants underwent an extensive medical examination by the attending endocrinologists as their regular practice for the assessment of diabetic complications.
Diagnosis of retinopathy: As per study protocol all study participants had undergone an extensive ophthalmologic examination including fundoscopy or retinal photography and measurement of visual acuity by the ophthalmologist as regular practice for the assessment of DR in tertiary care setting. The above information was collected from the medical records and documented in the case record form as either patient had DR or no DR. The diagnosis of nephropathy was confirmed by estimating 24 hours urine protein excretion of more than 500 mg/day. The presence of neuropathy was confirmed by physician if diagnosed with one or more abnormal finding of 10 gram monofilament, pinprick sensations and ankle reflexes.
The baseline characteristics such as age, gender, BMI, waist circumference, smoking status, alcoholic status, biochemical parameters, duration of diabetes, and complications of diabetes were presented as mean with standard deviation (SD) and numbers with percentages. Prevalence of retinopathy in each subgroup of patients categorized on the basis of duration of diabetes (0-20 years) was calculated.
Regression Model: The relationship between DR prevalence and T2DM duration was assessed by weighted linear regression (WLR) with weights for each year data being inversely proportional to binomial variance. This WLR method was adopted from an observational study conducted by Harris et al., to examine the relationship between diabetes duration and prevalence of retinopathy [5].
In a WLR method more weight is given to the observations with smaller variance because these observations provide more reliable information about the regression function than those with large variances. Initially, we fitted the regression model by unweighted least squares and residuals were analyzed. Then we have estimated the variance function or the standard deviation (SD) function by regressing either the squared residuals or the absolute residuals on the appropriate predictor. Then, we have used the fitted values from the estimated variance or SD function to obtain the weights (Wi). Weights were calculated by following equation.
Wi = 1/SD2
Then regression coefficients were estimated by using weights. To analyze the stability of regression model, 95% confidence intervals (CI) were obtained by inverse method.
Calculation of T2DM diagnosis lag: Initially, the period where DR prevalence was nil was estimated by back extrapolating the best- fitting equation correlating known T2DM duration with DR prevalence. This is the time from onset of retinopathy to clinical diagnosis of diabetes which was calculated as a point estimate by back extrapolating the intercept of the best-fitting regression line with the horizontal axis.
Since metabolic and biochemical changes attributable to diabetes are occurring in the eye that precede the development of detectable retinopathy. An estimate of minimum duration of diabetes necessary before evident retinopathy is made using data of Jarret et al., [19]. They had found that signs of DR began developing ~5 years after onset of diabetes. Thus, the period of time between onset of diabetes and detectable retinopathy, and that between detectable retinopathy and clinical diagnosis of T2DM, taken together, indicate the total diagnostic lag of T2DM. Earlier studies have also used the similar method to obtain an approximate idea about the undiagnosed period of diabetes [5, 6, 18]. We have used Microsoft excel and SPSS software to perform statistical analysis.
Results
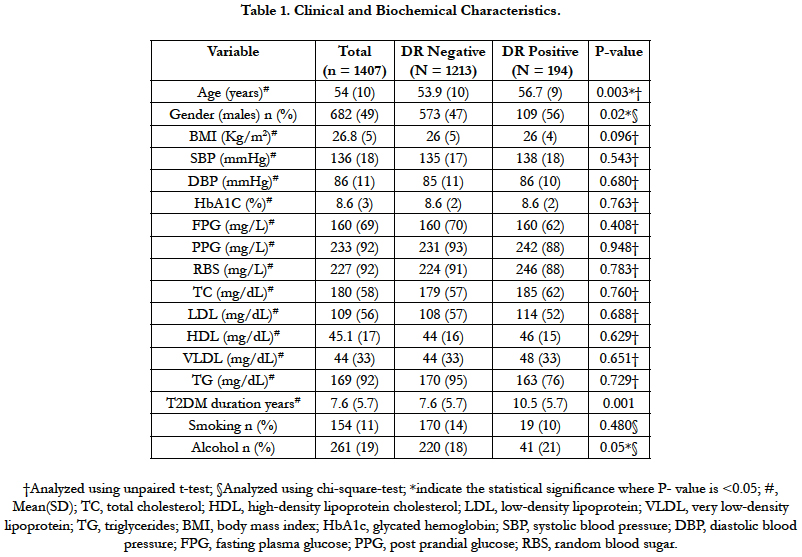
Of 1490 screened patients during the study period, 1407 (95%) were included in the present analysis after excluding patients with >20 years of T2DM. Average (SD) age of patients at study inclusion was 54.3 (10) years and 52% were females. The average (SD) duration of T2DM, BMI and HbA1C were 8 (5.8) years, 26.8 (4.7) Kg/m2 and 8.6% (2.3), respectively. 7.5% (n=106) patients were newly diagnosed with T2DM. 59.7% (n=92) were found to be smokers and 56% (n=146) were alcohol consumers. Table 1 show the clinical and biochemical characteristics of the study sample.
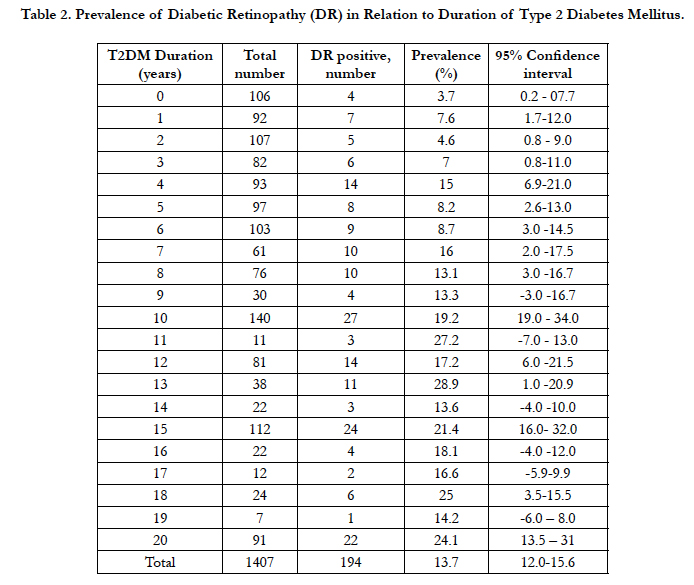
Out of 1407, 194 patients were diagnosed with DR at the time of clinical diagnoses of T2DM. Thus, the overall prevalence of DR was found to be 13.7% (95% CI, 12.08-15.69) (Table 2). Average (SD) age (56.7 (9) vs 53.9 (10), p-0.003) years and duration of T2DM (10.5 (5.7) vs 7.6 (5.7), p-0.001) of patients with DR was found to be significantly higher as compared to patients without DR. Higher proportion of males were found in DR positive group (56%) as compared to DR negative group (47%). However, the two groups were similar with respect to BMI, waist circumference, HbA1c and smoking status, alcohol consumption and other biochemical parameters (Table 1).
Table 2. Prevalence of Diabetic Retinopathy (DR) in Relation to Duration of Type 2 Diabetes Mellitus.
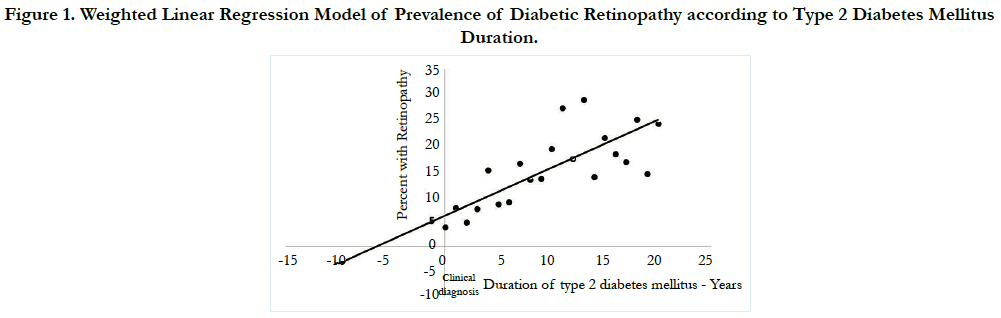
Figure 1 illustrates the fact that prevalence of DR increased linearly (R = 0.73, R2 = 0.68 p- <0.001) with duration of T2DM. On back extrapolating the regression line to horizontal axis the point at which DR prevalence is zero, we estimated the actual onset of time of DR in a subject to be 6.4 (95% CI, 4.5-10) years before the patients were clinically diagnosed with DR. The present weighted regression equation model to find the prevalence of DR is represented as follows.
Figure 1. Weighted Linear Regression Model of Prevalence of Diabetic Retinopathy according to Type 2 Diabetes Mellitus Duration.
Y= 0.0605 + 0.00944X
In addition to this 6.4 years period before clinical diagnosis of T2DM, metabolic and biochemical changes attributable to diabetes were occurring in the eye that precede the development of detectable retinopathy. The estimate of minimum duration of the DM which is necessary to pass before the emergence of DR was adopted from the data of Jarrett et al., [19]. In the study by Jarrett et al., 240 men with DM were prospectively followed for 10 years. During the first 5 years of follow up, 60 (25%) patients developed retinal lesions. Based on these findings, onset of DR is supposed to occur around 5 years after the onset of diabetes. Adding this minimum 5 years duration to the onset of DR (6.4 years) in the present data analysis, we found that the onset of T2DM may occur on an average 11.4 years (95% CI, 9.5-15.0) earlier than the clinical diagnosis of T2DM.
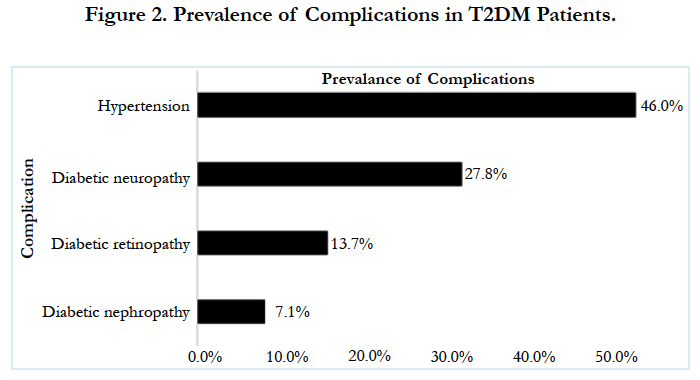
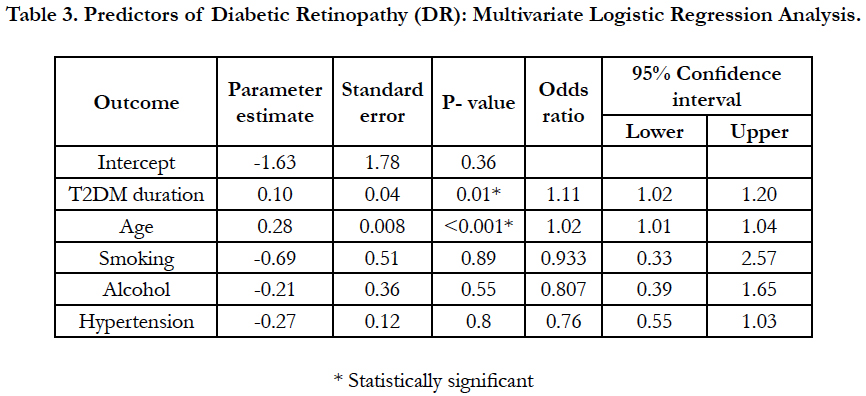
On multivariate logistic regression analysis duration of diabetes 1.11(95 CI%, 1.02-1.20, p-0.01) and age 1.02 (95 CI%, 1.01-1.04, p- <0.01)were the only two predictors found to be significant predictors of DR, while all other factors including smoking, alcohol and hypertension were not found to be significantly associated with DR (Table-3). About 68% variance in DR prevalence was explained by the duration of diabetes. Prevalence of other micro vascular complications such as neuropathy and nephropathy was found to be 27.8% (95% CI, 25.5-30.26) (n=392), 7.1% (95% CI, 5.8-8.5) (n=100), respectively. Among included subjects 46% (n=646) were found to be hypertensive.
Discussion
Retinopathy is usually the first observable micro vascular complication among the three specific complications of diabetes to develop namely; neuropathy, nephropathy and retinopathy. Retinopathy is a more sensitive and specific indicator than other micro vascular complications because the micro vascular structures in the eye can be directly examined at the earliest. Neuropathy and nephropathy are generally defined using arbitrary thresholds, and are less sensitive than retinal abnormalities [20].
Our findings from representative population showed that onset of retinopathy may appear 6.4 years before clinical diagnosis of DR in these populations. This indicates that retinopathy is detectable 6.4 years before T2DM is diagnosed. During this entire timeframe, from 6.4 years before until T2DM diagnosis, participants had diabetes and were developing retinopathy, yet their disease is totally untreated. This duration of 6.4 years is higher than earlier conducted studies by Thompson et al., (Egypt), Ramachandran et al., (south India) , Harris et al., (US and Australia) and Ellis et al., (UK) which reported the onset of DR to be 2.6, 4.1, 4-7 and 5.7 years before the clinical diagnosis was made, respectively [5, 12, 18, 20].
Evidence suggests that the onset of T2DM occurs probably earlier than the onset of DR. The estimate of minimum duration of the DM which is necessary to pass before the emergence of DR was adopted from the data of Jarrett et al., [19]. Adding the minimum 5 years duration to the onset of DR (6.4 years) from the onset of diabetic hyperglycemia in the present data analysis, we found that the onset of T2DM may occur on an average 11.4 years (95% CI, 9.5-15.4) earlier than the clinical diagnosis of T2DM.
Harris et al., attempted to estimate this delay between onset and clinical diagnosis of T2DM. they calculated the WLR between known duration and prevalence of DR in two different white populations (Wisconsin and Australian residents), and reported that DR developed 6.5 years (95% CI 4.1–9.9) and 4.2 years (95% CI 2.1–7.4) before diagnosis, respectively. They also added 5 years for retinopathy to develop after the onset of T2DM. This made the total duration of undiagnosed diabetes to 9–12 years [5]. Another study conducted by the same author in US reported that onset of DR occurred 6.5 years earlier than its clinical diagnosis, which are in line with the present study [4].
A study conducted by Ramachandran et al., in south Indian population estimated the duration of onset of DR to be 4.1 years preceding the diagnosis of Non insulin dependent diabetes mellitus (NIDDM). NIDDM was defined by an oral glucose load. Any retinopathy was assessed using a detailed dilated eye examination by an ophthalmologist. The overall prevalence of DR was lower than in previous reports. This estimate was lesser than the findings of present analysis [12].
In a study involving Egyptian patients using similar model, the onset of DR occurred 2.6 years (90% CI 0.3-8.4) prior to clinical diagnosis [20]. Adding 5 years for retinopathy to develop after the onset of diabetes, the total estimated time of undiagnosed diabetes duration comes to be 7.6 years.
A study conducted by Ellis et al., in UK estimated that the onset of detectable DR occurred 5.77 years (95% CI 4.6-7) before its diagnosis, which is also less than the present analysis [18].
A recent study by Porta et al., assessed the relationships between known duration of diabetes and prevalence of DR separately in insulin treated (IT) and non-IT patients. They reported that the onset of detectable DR occurred 8.46 years (using WLR model) and 3.89 years (using quadratic model) in non-IT patients, which is higher than present analysis [6].
These differences in the estimates may probably be due to different participant population characteristics in terms of definitions and methods (oral glucose load vs HbA1c vs FBS) to diagnose NIDDM and methods used for the detection of retinopathy. Severity of retinopathy is also not assessed in majority of the studies including the present one. The difference in the diagnostic lag among the studies may also be due to differences in knowledge about diabetes, availability of health care provisions, health policies, errors in screening of diabetes, changes in diagnostic criteria, difference in screening method, use of oral hypoglycemic and insulin and selection criteria of patients for the study. This diagnostic lag can impose huge burden of disease and its complications on the society and health policy makers. There is an urgent need to implement suitable early screening approaches at the community level and appropriate education of patients so that awareness among people about disease related morbidity and advantages of early diagnosis and treatment.
Limited studies have been conducted in India to assess the awareness about diabetes at community level. A cross sectional study conducted in Mangalore among the households showed that 35% subjects had average while 27.5% and 37.5% had poor and very poor awareness about diabetes and its complications, respectively [21]. Recent study including 16,607 subjects from four geographical regions of India namely Chandigarh, Tamil Nadu, Jharkhand and Maharashtra reported that 57% participants had not even heard of diabetes [22]. Another study among college students in south India reported that among 230 participants 53% had moderate knowledge awareness in general diabetic symptoms [23].
Evidence suggests that patients are not sufficiently equipped with the knowledge of diabetes, its associated complications and to manage their disease condition [21-23]. It indicates that there is a need of continued education, counseling of high risk population to increase the awareness of diabetes and reduction of diagnostic lag. A community based mass screening is suggested to be helpful in populations like ours with high prevalence of T2DM in order to reduce this diagnostic lag [24].
Strengths and Limitations
The strength of the present study lies in adequate sample size, representative population of T2DM including 7% newly diagnosed cases, adequate event rate (DR positive cases) and extensive ophthalmologic examination by experienced ophthalmologists. Limitations are experience from single center and back extrapolation of data. The strength of association between duration of diabetes and retinopathy is not high enough to explain percentage of variation. In addition, patients were lumped together irrespective of insulin consumption and any type of retinopathy.
Conclusion
The present analysis suggest that undiagnosed T2DM is not a benign condition. Significant morbidity in the form of high prevalence of retinopathy may present at clinically diagnosis of T2DM. Imprecise dating of disease onset also obscures investigations of the nature and importance of risk factors for diabetes complications as well as estimating the actual disease burden. Future studies must emphasize estimating the actual prevalence of disease and its complications and societal creation of diabetes awareness and knowledge.
Acknowledgements
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- International diabetic federation (IDF) Diabetic atlas 7th edition, Jan 2016.
- Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, et al., (2014) Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 103(2): 137-149.
- American Diabetes Association (2013) Executive summary: standards of medical care in diabetes-2013. Diabetes Care. 36(1): 4-10.
- Harris MI, Eastman RC (2000) Early detection of undiagnosed diabetes mellitus: a US perspective. Diabetes Metab Res Rev. 16(4): 230-236.
- Harris MI, Klein R, Welborn TA, Knuiman MW (1992) Onset of NIDDM occurs at least 4-7 yr before clinical diagnosis. Diabetes Care. 15(7): 815-819.
- Porta M, Curletto G, Cipullo D, Longrais R, Trento M, et al., (2014) Estimating the delay between onset and diagnosis of type 2 diabetes from the time course of retinopathy prevalence. Diabetes Care. 37(6): 1668-1674.
- Michael J Fowler (2008) Microvascular and Macrovascular Complications of Diabetes. Clinical Diabetes. 26(2): 77-82.
- Marglis S (2005) Diabetic microvascular complications: an overview. Adv Stud Med. 5(4A): 260-263.
- Bansal D, Gudala K, Muthyala H, Esam HP, Nayakallu R, et al., (2014) Prevalence and risk factors of development of peripheral diabetic neuropathy in type 2 diabetes mellitus in a tertiary care setting. J Diabetes Investig. 5(6): 714-721.
- Bansal D, Gudala K, Esam HP, Nayakallu R, Vyamusani RV, et al., (2014) Microvascular Complications and Their Associated Risk Factors in Newly Diagnosed Type 2 Diabetes Mellitus Patients. Int J Chron Dis. 2014: 1-7.
- World health organization (2002) Causes of blindness and visual impairment.
- Ramachandran A, Snehalatha C, Vijay V, Viswanathan M (1996) Diabetic retinopathy at the time of diagnosis of NIDDM in south Indian subjects. Diabetes Res Clin Pract. 32(1-2): 111-114.
- Raman R, Gupta A, Krishna S, Kulothungan V, Sharma T (2012) Prevalence and risk factors for diabetic microvascular complications in newly diagnosed type II diabetes mellitus. Sankara nethralaya diabetic retinopathy epidemiology and molecular genetic study (SN DREAMS, report 27). J Diabetes Complications. 26(2): 123-128.
- Rajalakshmi R, Amutha A, Ranjani H, Ali MK, Unnikrishnan R, et al., (2014) Prevalence and risk factors for diabetic retinopathy in Asian Indians with young onset type 1 and type 2 diabetes. J Diabetes Complications. 28(3): 291-297.
- Aggarwal RP, Ola V, Bishnoi P, Gothwal S, Sirohi P, et al., (2014) Prevalence of micro and macrovascular complications and their risk factors in type-2 diabetes mellitus. J Assoc Physicians Indi. 62(6): 504-508.
- Ohno T, Takamoto S, Motomura N (2008) Diabetic retinopathy and coronary artery disease from the cardiac surgeon's perspective. Ann Thorac Surg. 85(2): 681-689.
- Thomas RL, Dunstan F, Luzio SD, Chowdury S, Hale SL, et al., (2012) Incidence of diabetic retinopathy in people with type 2 diabetes mellitus attending the Diabetic Retinopathy Screening Service for Wales: retrospective analysis. BMJ. 344: e874.
- Ellis JD, Zvandasara T, Leese G, McAlpine R, Macewen CJ Baines PS, et al., (2011) Clues to duration of undiagnosed disease from retinopathy and maculopathy at diagnosis in type 2 diabetes: a cross-sectional study. Br J Ophthalmol. 95(9): 1229-1233.
- Jarrett RJ (1986) Duration of non-insulin-dependent diabetes and development of retinopathy: analysis of possible risk factors. Diabetic Med. 3(3): 261-63.
- Thompson TJ, Engelgau MM, Hegazy M, Ali MA, Sous ES, et al., (1996) The onset of NIDDM and its relationship to clinical diagnosis in Egyptian adults. Diabet Med. 13(4): 337-340.
- D'Souza P, Kundapur R, Udayakiran N (2015) A Cross sectional study to determine the prevalence of Diabetes Mellitus and its household awareness in the rural field practice areas of a medical college in Mangalore - A Pilot Study. NUJHS. 5(3): 43-46.
- Deepa M, Bhansali A, Anjana M, PradeepaM, Joshi S (2014) Knowledge and awareness of diabetes in urban and rural India: The Indian Council of Medical Research India Diabetes Study (Phase I): Indian Council of Medical Research India Diabetes. Indian J Endocrinol Metab. 18(3): 379–385.
- Raina, Dsouza, George Peter (2015) Diabetes awareness among college going students. IOSR J Den Med Sci. 14(6): 25-28.
- Basu S, Millett C, Vijan S, Hayward RA, Kinra S, et al., (2015) The health system and population health implications of large-scale diabetes screening in India: a microsimulation model of alternative approaches. PLoS med. 12(5): e1001827.