Obesity and Renal Prognosis (Assessed by Albuminuria and eGFR) in Type 2 Diabetics in Kinshasa
Makulo JR1*, Lepira FB1, Sumaili EK1, Mvitu MM2, Longo-Mbenza B3, Kintoki F4, Ngoy G4, Bukabau JB1, Mokoli V1, Longo A1, Nseka NM1, Jadoul M5
1 Division of Nephrology, Department of Internal Medicine, University Hospital of Kinshasa, Faculty of Medicine, University of Kinshasa, Kinshasa, Democratic Republic of Congo.
2 Department of Ophthalmology, University Hospital of Kinshasa, Faculty of Medicine, University of Kinshasa, Kinshasa, Democratic Republic of
Congo.
3 Faculty of Health Sciences, Walter Sisulu University, Eastern Cape, South Africa.
4 Division of Cardiology, Department of Internal Medicine, University Hospital of Kinshasa, Faculty of Medicine, University of Kinshasa, Kinshasa,
Democratic Republic of Congo.
5 Division of Nephrology, Cliniques universitaires Saint-Luc, Université catholique de Louvain, Brussels, Belgium.
*Corresponding Author
Jean-Robert Makulo, MD PhD,
Division of Nephrology, Department of Internal Medicine,
University Hospital of Kinshasa, Faculty of Medicine,
University of Kinshasa, Kinshasa, Democratic Republic of Congo.
Tel: 00243990205367
E-mail: jrmakulo@yahoo.fr
Received: December 08, 2015; Accepted: January 29, 2016; Published: January 30, 2016
Citation: Makulo JR et al., (2016) Obesity Associated and Renal Prognosis (Assessed by eGFR and Albuminuria) in Type 2 Diabetics in Kinshasa. Int J Diabetol Vasc Dis Res,. 4(1), 140-145.DOI : dx.doi.org/10.19070/2328-353X-1600030
Copyright: Makulo JR© 2016. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Obesity is associated with greater survival in end stage renal disease (ESRD).
Objective: To assess the association between cardiovascular risk factors and renal Prognosis, assessed by eGFR and albumin/ creatinine ratio (ACR), in Congolese Type 2 diabetic patients (T2DM).
Methods: Cross-sectional study that included T2DM followed in the University hospital of Kinshasa. ACR and eGFR were used to assess the renal prognosis according to the KDIGO2012 guideline. A Logistic regression model was used to identify independent determinants of renal prognosis.
Results: Patients (53 % men, 4.5 % in ESRD) were aged 56 ± 11 years and had a median duration of diabetes of 4 years (IQ: 1-10 years). In the whole group, 40.9 % had a low renal risk prognosis versus 24.9 % a moderately increased risk; 13.3 % a high risk and 21.0 % a very high risk. Diabetes duration ≥ 5 years (aOR: 8.69; p < 0.001), SBP > 130 mmHg (ORa: 15.87; p < 0.001), abdominal obesity (aOR: 0.28; p = 0.036) and HbA1c ≥ 64 mmol/mol (8%)(aOR: 0.13; p < 0.001) emerged as independent determinants of very high renal risk.
Conclusions: While obesity in the present study was paradoxically associated with low renal risk, a reverse epidemiology feature, higher SBP and long duration of diabetes emerged as the main determinants of renal prognosis.
2.Introduction
3.Materials and Methods
3.1.Patients
3.2.Interview and clinical examination
3.3.Laboratory tests
3.4.Renal prognosis
3.5.Statistical Analysis
4.Results
4.1.Study population and baseline characteristics
4.2.Frequency and Prognosis of CKD in the study population
4.3.Determinants of Renal prognosis
5.Discussion
6.Conculsion
7.Author Contributions
8.Acknowledgements
9.References
Key Words
CKD; Obesity; Diabetes; Renal Prognosis; KDIGO.
Introduction
Obesity is a growing public health problem worldwide. In some regions, it affects 50 percent of the general population [1]. In fact, obesity has been recognized per se as a chronic disease and it contributes to the increased incidence of diabetes, hypertension and cardiovascular disease [2]. In 2010, overweight and obesity were estimated to cause 3.4 million deaths worldwide [2]. Moreover, obesity is associated with an increased risk of chronic kidney disease (CKD). Indeed, most studies indicate a direct relationship between body mass index (BMI) and CKD risk [3-5]. The detrimental effect of obesity on kidney outcome is undisputable in CKD patients not on dialysis. However, survival in overweight and obese patients on chronic dialysis is paradoxically better than in patients with a normal BMI [6]. A similar protective role has been described for high serum creatinine [7]. The presence of the "malnutrition-inflammation complex syndrome" in dialysis patients may explain this reverse epidemiology [7].
In sub-Saharan, Africa (SSA), some studies in the general population and in diabetic patients have already established the detrimental effect of obesity on the kidney [8-11]. Indeed, these studies have shown that obesity increases the risk of albuminuria and renal failure. However, they did not assess whether obesity in Type 2 diabetic patients (T2DM) was associated with ESRD. In order to assess the role of obesity in Congolese Type 2 diabetic patients (T2DM), this study was performed. It aimed to identify cardiovascular risk factors that are significantly associated with a moderately poor to very poor renal prognosis, as assessed by eGFR and albumin/creatinine ratio (ACR) [12].
From July 1st 2007 to December 30th 2007, consecutive T2DM black patients followed at the Division of Diabetes and Metabolic diseases of the department of internal medicine, University hospital of Kinshasa, Democratic Republic of Congo (DRC) were invited to participate in the present cross-sectional study. They all provided written consent. Type 2 diabetes was defined according to the American Diabetes Association 2003 criteria (ADA) [13].
Sex, age, duration of diabetes, past and present medications (insulin, oral hypoglycemic agents), lifestyle, personal or family history of hypertension, antihypertensive agents, tobacco and /or alcohol habits and lipid-lowering therapy were recorded. Anthropometric measurements were obtained for weight, height and waist circumference (WC). Body mass index (BMI) was used to define obesity (BMI ≥ 30 kg/m²), overweight (BMI 25-29 kg/ m²), normal weight (18.5-24 kg/m²) and underweight (<18.5 kg/m²) [14]. Abdominal obesity was defined as WC ≥ 94 cm in men and ≥ 80 cm in women [15]. Three measurements of blood pressure were performed at 5 minute-intervals using a mercury sphygmomanometer Riester®, and the average of the last two measures was used. Hypertension was defined as SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg or antihypertensive treatment.
Capillary fasting glucose (CFG) was measured with a portable Glucocard X-meter® device (Menarini diagnostics, Zaventen, Belgium). HbA1c was measured on capillary blood using a Bayer DCA 2000® device (Bayer AG, Leverkusen, Germany) which uses an enzyme-linked immunosorbent assay. Assays of total cholesterol, HDLc and triglycerides (TG) were also performed on capillary blood using Cholestech LDX® device (Med Tek, Salt lake city, Utah, USA) whose principle is based on enzymatic reactions in series. The rate of LDLc was calculated using the Friedelwald formula which is integrated in the software of Cholestech LDX® device [16]. The thresholds defining lipid disorders were: total cholesterol > 5.18 mmol/l, LDLc > 3.37 mmol / l, HDLc < 1.04 mmol / l for men and < 1.30mmol/l for women and TG > 1.71mmol/l [17]. Assays for serum creatinine were performed using Genesys 20® spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), the kinetic Jaffe method and reagents were manufactured by Biomerieux®. We calibrated the creatinine results measured with the Jaffe method against a traceable isotope dilution mass spectrometry (IDMS) enzymatic method as described elsewhere [10]. The Glomerular Filtration Rate (GFR) was estimated by the abbreviated "Modification of Diet in Renal Disease study (MDRD) formula [18]. According to the KDIGO 2012CKD guideline, GFR was categorized as grade 1, 2, 3a, 3b, 4 and 5 [12].
Mid-stream urine samples were collected into a sterile jar in the morning, before the lunch. For women with menses, the samples were taken one week after the end of menstruation. We first tested all urine samples with the semi-quantitative Combi 9® strips (Nagel, Duren, Germany). In case of samples containing blood, white cells or nitrates, additional analyses including bacteriological tests were performed. Patients with culture proven urinary tract infection were prescribed antiseptics/antibiotics before a second Combi 9 test. Urinary albumin and creatinine measurements were performed by an immuno-assay method using the Bayer DCA 2000® device (Bayer AG, Leverkusen, Germany). The albumin excretion rate was expressed in mg/l and creatinine excretion rate in g/l. Albuminuria A1, albuminuria A2 and albuminuria A3 were defined as albumin to creatinine ratio (ACR) < 30 mg/g, 30 to 299 mg/g and ≥ 300 mg/g, respectively [19].
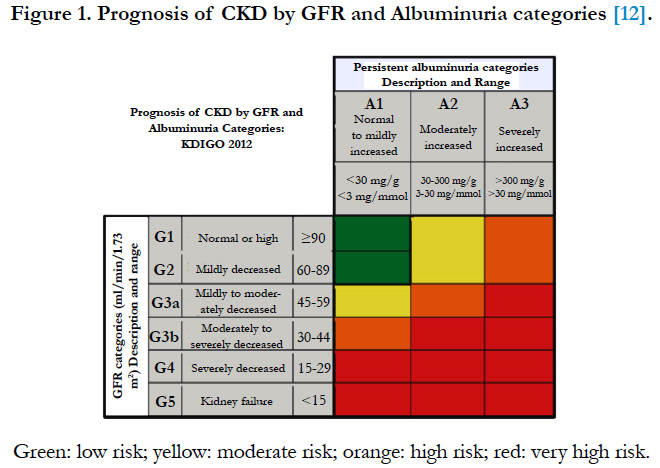
According to GFR and ACR categories, four categories of renal prognosis were defined: low risk; moderately increased risk; high risk and very high risk (Figure 1) [12].
Analyses were performed using SPSS Version 21.0 (SPSS Inc, Chicago, Il, USA). Data are presented as frequency, percentage, median with interquartile (IQ), mean ± standard deviation (SD). Chi-square test, Student-t test, U Mann-Whitney test, ANOVA and Kruskal-Wallis test were used for comparison of proportions and means, where appropriate. Logistic regression analysis was then performed to identify independent determinants of moderately increased risk -high risk and very high renal -risk. A p value < 0.05 indicated statistical significance.
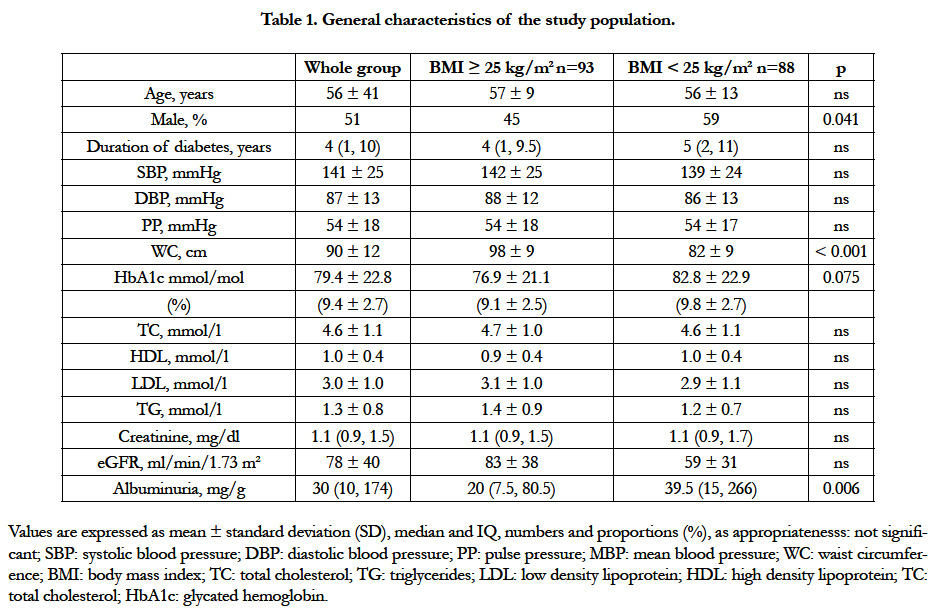
The 181 included T2DM patients (53% males; aged 56 ± 11 years) had a known median duration of diabetes of 4 years (IQ: 1-10 years). HbA1c was 79 ± 6 mmol/mol (9.4 ± 2.7%). Only 64 patients (35%) had HbA1c < 64 mmol/mol (8%) and 36 patients (20%) had HbA1c < 53 mmol/mol (7%). Seventy nine patients (44%) were hypertensive and all of them were taking blood pressure-lowering drugs. 60% of hypertensive patients received a calcium channel blocker (CCB), 18% a diuretic, 9% a CCB combined with a diuretic, 9% a angiotensin convertingenzyme inhibitor (ACEI) and 4% an ACEI in combination with a diuretic. The overall frequency of overweight was 32.6% versus 18.8% for obesity (grade 1=14.9%, grade 2=3.3%; grade 3:0.6%), with a lower prevalence in men than women (13% versus 26%, p<0.017). Abdominal obesity (54% in the whole group) was also less frequent in men (30%) than women (81%) (p<0.001). High levels of total cholesterol, LDLc and TG were observed in 31.9%, 38.9% and 16.2% of patients respectively. HDLc was low in 78.1% of patients. Only 25 patients (6.6%) were receiving statins and 55 patients (14.4%) an anti-platelet agent. Smoking and alcohol consumption were reported in 8.7 and 28.5% of patients, respectively. Other clinical and biological data are detailed in Table 1.
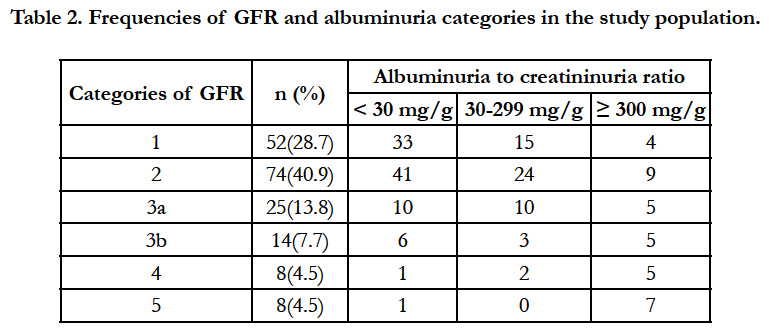
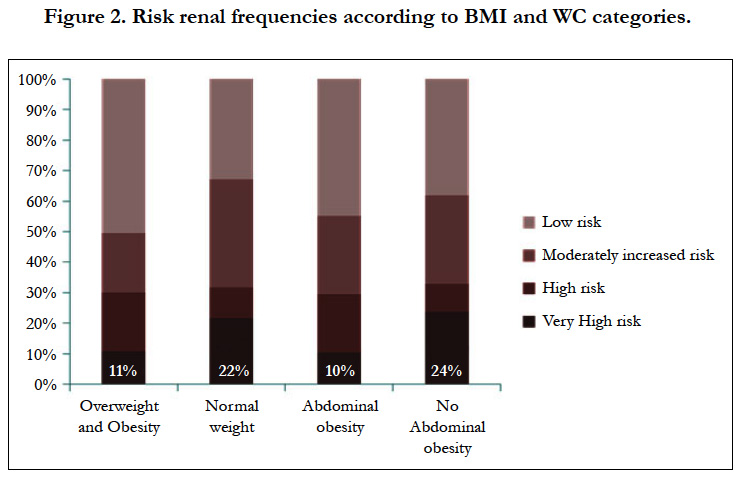
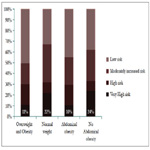
The relative frequencies of each category of GFR and albuminuria are listed in Table 2. In total, one hundred and seven patients (58.9%) had CKD while 74 patients (40.9 %) had albuminuria A1 with GFR > 60 ml/min/1.73 m2 (no CKD). As regards the renal prognosis, 74 patients (40.9%) had a low risk versus 45 patients (24.9%) a moderately increased risk; 24 patients (13.3%) a high risk and 38 patients (21.0%) a very high risk. Figure 2 shows that a very high renal risk was less frequent in overweight and obese patients.
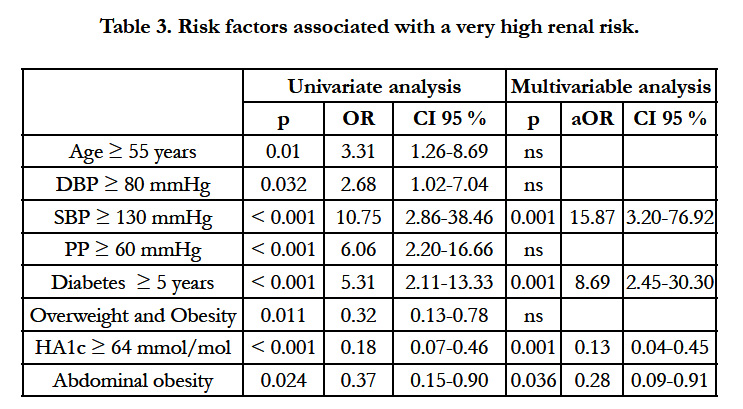
The determinants of very high renal risk are depicted in table 3. In multivariable analysis, duration of diabetes ≥ 5 years and systolic blood pressure (SBP)>130 mmHg were associated with a significantly increased risk whereas abdominal obesity and HA1c ≥ 64 mmol/mol (8%) tended be associated with a lower risk. Although overweight and global obesity were significantly associated with very high risk in univariate analysis, the association did not persist in multivariable analysis. Lipid abnormalities (total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides) were not associated with a very high renal risk.
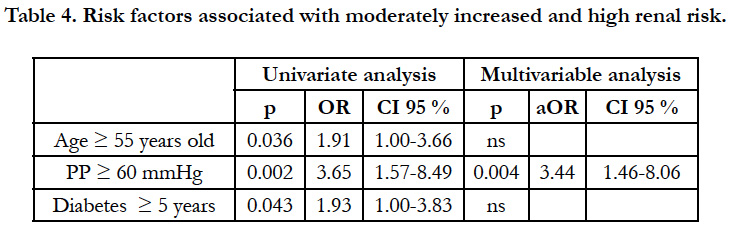
Pulse pressure (PP) > 60 mmHg emerged as the single determinant of moderately increased and high renal risk (Table 4). Once again, abnormalities of body weight and lipid disorders were not associated with a moderately increased renal risk.
Discussion
The present study showed that in T2DM subjects in DRC, abdominal obesity and high HbA1c were associated with a lower frequency of very high renal risk whereas high SBP, long duration of diabetes and high PP were associated with a worse renal prognosis. Lipid abnormalities, obesity and overweight were not associated with renal prognosis. Moreover, many patients were at highor very high renal risk.
Renal prognosis is evaluated on the basis of albuminuria and glomerular filtration rate, two parameters strongly associated with progression to end stage renal disease (ESRD) [20]. On the other hand, it is known that relatively few patients with CKD progress to ESRD and many of them die, mostly from cardiovascular disease prior to dialysis [21, 22]. The inverse association between abdominal obesity and very high renal risk is in line with the reverse epidemiology literature in ESRD patients [6-8]. In practice, these data suggest that there is probably no benefit to recommend weight loss in T2DM with CKD already established. Possible causes of the reverse epidemiology in ESRD and in dialysis patients include competitive risk factors (malnutrition versus overnutrition), role of micronutrients, protein-energy wasting and inflammation, hemodynamic instability, alteration of circulatory cytokines, sequestration of uremic toxins in adipose tissue, and endotoxin-lipoprotein interaction [8]. However, according to recent data from the United States, being overweight is associated with a significantly lower mortality as compared with the normal weight category in the general population [23]. Relative to normal weight, both obesity (all grades) and grades 2 and 3 obesity were associated with significantly higher all-cause mortality. Grade 1 obesity overall was not associated with higher mortality, and overweight was associated with significantly lower all-cause mortality [23]. The use of predefined standard BMI groupings can facilitate between-study comparisons. In sub-Saharan Africa (SSA), specifically in Nigeria, Ulasi et al reported that BMI and WC are negatively correlated with albuminuria [24]. The fact that the majority of T2DM obese patients in our study had obesity grade 1, may in part explain our results. It is therefore important to conduct studies with a larger sample that can categorize the different grades of obesity and central obesity in order to assess their implications for CKD prognosis.
We found an inverse association between HbA1c and a very high renal risk. This can probably be explained by the cross-sectional design of our study: indeed renal filtration and catabolism of insulin fall in renal insufficiency with a resultant tendency to hypoglycaemia. In contrast, in a recent Prospective cohort analysis of 11,357 american subjects (773 with a history of diagnosed diabetes), Selvin E et al showed that categories of HbA1c were associated with the risk of CKD, with adjusted hazard ratios (HRs) of 1.12 (0.94-1.34) and 1.39 (1.04-1.85) for HbA1c 5.7-6.4% and ≥ 6.5%, respectively, as compared with <5.7%. The corresponding HRs for ESRD were 1.51 (0.82-2.76) and 1.98 (0.83-4.73), respectively (p for trend = 0.047) [25].
The results related to SBP and PP corroborate literature data which indicate that in T2DM, SBP is a stronger predictor than DBP of renal outcomes and a high baseline PP a strong risk factor for CKD progression [26].
Our study has some limitations that must be acknowledged. First, our results can not be generalized to all T2DM since it is not a multicentric study and moreover, the sample size is not very large. Second, the study site (university hospital), may lead to underestimate the renal prognosis, due to the expected referral bias of more severe cases. Thirdly, the search for risk factors of any disease ideally requires a cohort study rather than a crosssectional study as is the case in this study. This is especially true for abnormalities of body weight that were associated with a lower renal risk. It is also possible that patients with lower BMI or WC have lower socioeconomic status and some comorbidities that would explain that they have a worse CKD prognosis compared to overweight and obese patients. Fourth, both eGFR and ACR were assessed only once, thus not meeting the KDIGO definition of CKD, but this has been the case in many other studies [27, 28], and might change individual results but not those of a group of patients.
Conclusion
While obesity in the present study was paradoxically associated with low renal risk, a reverse epidemiology feature, higher SBP and long duration of diabetes emerged as the main determinants of renal prognosis in T2DM patients in RDC.
Author Contributions
JRM designed, acquired data, analyzed, interpreted data, drafted and revised the manuscript. FK, BJB, GN, BLM, FBL,VM, EKS, AL, MMN and MJ analyzed, interpreted data and revised the manuscript. MVM acquired data and revised the manuscript.
Acknowledgements
This work was supported by the Vlaamse Interuniversitaire Raad (VLIR) and Flemish cooperation project proponent VLIR-diabetes. We thank Professor Eric Muls Department of Endocrinology, Katholieke Universiteit Leuven, who initiated the "VLIR- Diabetes" project in DR Congo.
References
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. (2014) Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384(9945): 766-781.
- Laville M (2011) Renal consequences of obesity. Nephrol Ther 7(2): 80-85.
- Kopple JD (2010) Obesity and chronic kidney disease. J Ren Nutr 20(5 Suppl): S29-30.
- Kramer H (2006) Obesity and chronic kidney disease. Contrib Nephrol 151: 1-18.
- Kato S, Nazneen A, Nakashima Y, Razzaque MS, Nishino T, et al. (2009) Pathological influence of obesity on renal structural changes in chronic kidney disease. Clin Exp Nephrol 13(4): 332-340.
- Jialin W, Yi Z, Weijie Y (2012) Relationship between body mass index and mortality in hemodialysis patients: a meta-analysis. Nephron Clin Pract 121(3-4): c102-111.
- Park J, Ahmadi SF, Streja E, Molnar MZ, Flegal KM, et al. (2014) Obesity paradox in end-stage kidney disease patients. Prog Cardiovasc Dis 56(4): 415-425.
- Sumaili EK, Krzesinski JM, Zinga CV, Cohen EP, Delanaye P, et al. (2009) Prevalence of chronic kidney disease in Kinshasa: results of a pilot study from the Democratic Republic of Congo. Nephrol Dial Transplant 24(1): 117-122.
- Kaze FF, Halle MP, Mopa HT, Ashuntantang G, Fouda H, et al. (2015) Prevalence and risk factors of chronic kidney disease in urban adult Cameroonians according to three common estimators of the glomerular filtration rate: a cross-sectional study. BMC Nephrol 16: 96.
- Makulo JR, Nseka MN, Jadoul M, Mvitu M, Muyer MT, et al. (2010) Albuminuria during the screening for diabetes in a semi-rural area (Kisantu City, DR Congo). Nephrol Ther 6(6): 513-519.
- Noubiap JJ, Naidoo J, Kengne AP (2015) Diabetic nephropathy in Africa: A systematic review. World J Diabetes 6(5): 759-773.
- Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, et al. (2011) The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference Report. Kidney Int 80(1): 17-28.
- Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (1997) Diabetes Care 20(7): 1183-1197.
- World Health Organization (2000) Obesity: preventing and managing the global epidemic. Report on a WHO consultation. World Health Organ Tech Rep Ser 894.
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, et al. (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120(16): 1640-1645.
- Friedelwald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low density lipoprotein in plasma without the use of the preparative ultracentrifuge. Clin Chem 18(6): 499-502.
- Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, et al. (2008) Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care 31(4): 811-822.
- Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, et al. (2006) Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145(4): 247-254.
- Bennett PH, Haffner S, Kasiske BL, Keane WF, Mogensen CE, et al. (1995) Screening and management of microalbuminuria in patients with diabetes mellitus: recommendations to the Scientific Advisory Board of the National Kidney Foundation from an ad hoc committee of the Council on Diabetes Mellitus of the National Kidney Foundation. Am J Kidney Dis 25(1): 107- 112.
- Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, et al. (2011) Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 79(12): 1331-1340.
- Demoulin N, Beguin C, Labriola L, Jadoul M (2011) Preparing renal replacement therapy in stage 4 CKD patients referred to nephrologists: a difficult balance between futility and insufficiency. A cohort study of 386 patients followed in Brussels. Nephrol Dial Transplant 26(1): 220-226.
- Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH (2004) Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 164(6): 659- 663.
- Flegal KM, Kit BK, Orpana H, Graubard BI (2013) Association of all-cause mortality with overweight and obesity using standard body mass Index categories. A systematic review and meta-analysis. JAMA 309(1): 71-82.
- Ulasi I, Ijoma C, Arodiwe E, Okoye J, Ifebunandu N (2009) Lifestyle Risk Factors: Do they contribute to chronic kidney disease in developping countries? The Internet Journal of Nephrology 6(1).
- Selvin E, Ning Y, Steffes MW, Bash LD, Klein R, Wong TY, et al. (2011) Glycated hemoglobin and the risk of kidney disease and retinopathy in adults with and without diabetes. Diabetes 60(1): 298-305.
- Bakris GL, Weir MR, Shanifar S, Zhang Z, Douglas J, et al. (2003) Effects of blood pressure level on progression of diabetic nephropathy: results from the RENAAL study. Arch Intern Med 163(13): 1555-1565.
- Inker LA, Coresh J, Levey AS, Tonelli M, Muntner P (2011) Estimated GFR, Albuminuria, and Complications of Chronic Kidney Disease. J Am Soc Nephrol 22(12): 2322-2331.
- Barreto SM, Ladeira RM, Duncan BB, Schmidt MI, Lopes AA, et al. (2015) Chronic kidney disease among adult participants of the ELSA-Brasil cohort: association with race and socioeconomic position. J Epidemiol Community Health.