Recent Advances Of Metal Nanoparticles Applications In Soft And Hard Tissue Regeneration: Dental Overview
Mona El Deeb*, Amany A. Rabea, Yasmin M. Elghazawy, Esraa G. Hassan, Aya M. Reyad
Faculty of Oral and Dental Medicine, Oral Biology, Future University in Egypt, Cairo, Egypt.
*Corresponding Author
Mona El Deeb,
Faculty of Oral and Dental Medicine, Oral Biology, Future University in Egypt, Cairo, Egypt.
Tel: +20 1005000490
E-mail: mona.eldeeb@fue.edu.eg
Received: February 17, 2022; Accepted: March 06, 2022; Published: March 09, 2022
Citation: Mona El Deeb, Amany A. Rabea, Yasmin M. Elghazawy, Esraa G. Hassan, Aya M. Reyad. Recent Advances Of Metal Nanoparticles Applications In Soft And Hard Tissue Regeneration: Dental Overview. Int J Dentistry Oral Sci. 2022;9(2):5254-5263. doi: dx.doi.org/10.19070/2377-8075-220001054
Copyright: Mona El Deeb©2022. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Nanoparticles are solid colloidal particles showing unique physical and chemical features. Metallic nanoparticles (MNPs) are broadly used in biomedical therapeutic applications and tissue engineering. They have gained attention due to their massive potential in nanotechnology. In this review, we aimed to highlight the recent updates of MNPs applications in hard and soft tissue regeneration from histological point of view. Researches revealed enhanced osteogenic differentiation potential of MNPs, successful remineralizing effect on dental hard tissues, together with effective periodontal ligament regeneration and accelerated wound healing process.
2.Introduction
3.Materials and Methods
3.Results
4.Discussion
5.Conclusion
5.References
Keywords
Metal Nanoparticles; Bone Regeneration; Dental Tissue Regeneration; Supporting Tissue Regeneration; Wound Healing.
Abbreviations
METALLIC NANOPARTICLES (MNPS); NANOPARTICLES (NPS); GOLD NANOPARTICLES
(AUNPS); SILVER NANOPARTICLES (AGNPS); IRON OXIDE NANOPARTICLES (IONPS); ALUMINUM NANOPARTICLES
(ALNPS); COPPER NANOPARTICLES (CUNPS); ZIRCONIUM NANOPARTICLES (ZRNPS); TITANIUM
DIOXIDE NANOPARTICLES (TIO2NPS); ZINC OXIDE NANOPARTICLES (ZNONPS); CERIUM OXIDE
NANOPARTICLES (CEO2NPS); EXTRACELLULAR MATRIX (ECM); TISSUE ENGINEERING (TE); HUMAN
MESENCHYMAL STEM CELLS (HMSCS); HUMAN BONE MARROW MESENCHYMAL STEM CELLS (HBMMSCS);
REACTIVE OXYGEN SPECIES (ROS); POLYETHYLENEIMINE (PEI); ALKALINE PHOSPHATASE (ALP);
OSTEOCALCIN (OCN); OSTEOPONTIN (OPN); RUNT-RELATED TRANSCRIPTION FACTOR 2 (RUNX2); TETRAMETHYLPIPERIDINE-
N-OXYL (TEMPO); PERIODONTAL LIGAMENT STEM CELL (PDLSC); HYPOXIAINDUCIBLE
FACTOR 1? (HIF1?); INTERLEUKIN 8 (IL8); SILVER HYDROXYAPATITE (AG-HA); SILK FIBROIN
(SF); MITOGEN-ACTIVATED PROTEIN KINASE (MAPK); HUMAN SERUM ALBUMIN (HSA); FIBROBLAST
GROWTH FACTOR 2 (FGF2); VASCULAR ENDOTHELIAL GROWTH FACTOR (VEGF); CHITOSAN (CS); BONE
MORPHOGENIC PROTEIN (BMP); NANO SILVER FLUORIDE (NSF); POLYETHYLENE GLYCOL (PEG); DENTAL
PULP STEM CELLS (DPSCS); PERIODONTAL LIGAMENT FIBERS (PDL); POLYCAPROLACTONE (PCL).
Introduction
Human tissues consist of highly organized and specialized cells
associated with extracellular matrix (ECM). This offers support
for cellular adhesion and proliferation, as well as regulation of
intercellular communication [1]. Regenerative medicine based on
tissue engineering (TE) depends onsetting up systems that restore,
replace or regenerate destroyed tissues [2].
Nanoparticles (NPs) have exciting properties as they interact with
living systems, and possess variable characteristics which provide
biocompatibility and circumstantial bioactivity [3, 4]. Small size
of NPs, similar to ECM components, and their large surface to
volume ratiomake them compatible to be used in TE [5]. Different
types of NPs are available such as ceramic, metallic, carbonbased
and composite-based [6]. Metallic nanoparticles (MNPs)
are biocompatible materials nevertheless, they necessitate long
term cytotoxicity testing [7]. They have variable applications in biomedicine, due to their unique physicochemical features, such
as high energy atoms [8], high ratio of surface area-to-volume
and high surface energy [9]. MNPs can be prepared with different
chemical, physical or biological methods [10].
With the rapid progress of nanotechnology, nanomaterials such
as NPs, nanofibers, nanotubes, and others have been widely used
in dental and cranio-maxillofacial TE. We summarized here the
recent updates of nanometals’applications in dental hard and soft
tissues.
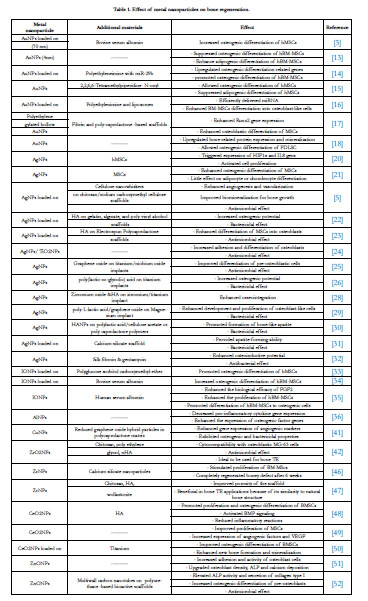
Bone Regeneration
Gold nanoparticles (AuNPs)
It is proved that there is a link between AuNPs and osteogenic
differentiation of stem cells due to their shape, size, and surface
properties. A study reported that AuNPs of size 70 nm could
increase the osteogenic differentiation of human mesenchymal
stem cells (hMSCs) [11] by affecting Wnt/ß-catenin and p38 signaling
pathways [12]. However, small sized AuNPs (4nm) suppress
osteogenic differentiation, but enhance adipogenic differentiation
of human bone marrow mesenchymal stem cells (hBM-MSCs).
This could be related to reactive oxygen species (ROS) production
by the small sized AuNPs [13].
In hBM-MSCs and MC3T3-E1 cells, miR-29b-delivered polyethyleneimine
(PEI)-capped AuNPs ef?ciently promoted osteogenic
differentiation by enhancing the expression of alkaline
phosphatase (ALP), osteocalcin (OCN), osteopontin (OPN), and
Runt-related transcription factor 2 (Runx2)[14]. Also, 2,2,6,6-Tetramethylpiperidine-
N-oxyl (TEMPO) conjugated AuNPs allowed
osteogenic differentiation of human MSCs, though suppressing
the adipogenic differentiation [15].
AuNPscan act as vehicles to deliver miRNA to permit the growth
and differentiation of BM-MSCs [16], and enhance Runx2 gene
expression, thusfacilitating their osteoblastic differentiation [17].
Moreover, AuNPs allows osteogenic differentiation of periodontal
ligament stem cell (PDLSC) and osteoid tissue formationthrough
enhancing bone-related protein expression and mineralization
[18].(Table 1)
Silver nanoparticles (AgNPs)
Silver based scaffolds are highly efficient due to their good adhesion
and spreading potential on cells. They also exhibit antibacterial
activity, enhanced osteogenic ability and proliferative effect on
osteoblasts [19].
AgNPs could combine with DNA of human MSCs, and trigger
the expression of hypoxia-inducible factor 1a (HIF1a) and interleukin
8 (IL 8) gens that activate cell proliferation [20]. An in vivo
study of mouse model showed that AgNPs enhanced osteogenic
differentiation of MSCs, but had very little effect on adipocyte or
chondrocyte differentiation [21]. Ongoing, Hasan et al (2018) [5]
reported that AgNPs modified scaffolds possess appropriate porosity
for bone TE applications by promoting vascularization and
osteogenesis. Scaffolds embedded with silver hydroxyapatite (Ag-
HA) nanoparticles showed continued release of metallic ions, and
efficient adhesion and osteogenic potency on mammalian cells
[22, 23].
Additionally, nanotubular titanium oxide coated with Ag nanowires
appeared to be suitable for osteoblast-like cells adhesion and
proliferation, together with apatite crystal formation [24]. Similar
data were demonstrated for titanium implants coated with AgNPs
[25, 26]. Again, HA coating by nanosilver promoted osseointegration
of implants [27, 28]. Moreover, electrospun composites
with AgNPs increased the formation of bone-like apatite [29, 30],
while calcium silicate scaffold and silk fibroin (SF) incorporated
AgNPs showed bactericidal propertiesand osteogenic effect [31,
32].(Table 1)
Iron nanoparticles
Iron oxide nanoparticles (IONPs) promote osteogenic differentiation
of stem cells. Polyglucose sorbitol carboxymethyl-ether
(PSC) coated IONPs are structurally stable in hMSCs and allowtheir
osteogenic differentiation in vitro through activation ofclassical
mitogen-activated protein kinase (MAPK) signal pathway
[33].
However, stimulation of IONPs-loaded bovine serum albumin
showed high uptake rate into hBM-MSCs withincreased osteogenic
differentiation [34]. Moreover, coating of IONPs with human
serum albumin (HSA) permitted its binding to fibroblast
growth factor 2 (FGF2) and differentiation of hBM-MSCs into
bone cells[35].(Table 1)
Aluminum nanoparticles (AlNPs)
Chen et al. (2017) [36] investigated the osteogenic potential of
macrophage cell culture on nanotopographic aluminum. BMMSCs
interacted with the nanoporous and showed that the spread
of cells was influenced by pore size. This resulted indecreased
pro-inflammatory cytokine gene expression and ROS together
with increased mineralization, OPN and collagen type I expressions.(
Table 1)
Copper nanoparticles (CuNPs)
Copper has the ability to stimulate collagen fiber deposition and
angiogenesis [37]. It induces the differentiation of mesenchymal
cells to the osteogenic lineage [38] and treatsosteoporosis [39, 40]
by enhancing angiogenic activity and gene expression of vascular
endothelial growth factor (VEGF) and angiogenic growth factor
2 [41]. So,CuNPs have been considered as new additives in bone
TE [42].(Table 1)
Zirconium nanoparticles (ZrNPs)
Zirconium (Zr) is considered as an osteoinductive and biocompatible
material of low cytotoxicity [43, 44]. Bhowmick et al. (2017)
[45] synthesized a composite scaffold containing zirconium oxide
nanoparticles, which showed ideal properties for bone TE due to
its resemblance to cancellous bone. Further, Doostmohammadi
et al. (2019) [46] fabricated zirconium modified nanoparticles
that stimulated the proliferation of BM-MScs in a boney defect,
showing complete regeneration after 6 weeks. Additionally, Maghsoudlou
et al. (2020) [47] designed a biodegradable nanocomposite
containing chitosan (CS), HA, and ZrNPs that improved the
porosity of the scaffold. (Table 1)
Nanoceria
The incorporation of cerium oxide nanoparticles (CeO2NPs) into
a HA covering allowed motivation of bone morphogenic protein
(BMP) signaling [48]. Also, bone scaffold containingCeO2NPs
helped the proliferation of MSCs by increasing intracellular calcium
level and expression level of angiogenic factors and VEGF
[49]. Li et al. (2018) [50] deposited CeO2NPs on titanium surface
to detect the biological response of new bone formation. The
prepared NPs were in mixed Ce3+/Ce4+ valence state, and the increase
in the surface Ce3+/Ce4+ ratio enhanced new bone formation
and mineralization. The valence promoted bone regeneration
with no need for exogenous osteogenic inducer.(Table 1)
Zinc nanoparticles
Human osteoblasts were cultured onnano-sized zinc oxide (ZnO)
ceramic compacts. The adhesion and activity of osteoblast
cells were signi?cantly raised with upgraded osteoblast density,
ALP and calcium mineral deposition. Consequently, this allows
ZnONPs to be promising in application for orthopedic implants
[51]. Furthermore, the incorporation of ZnONPs, with multiwall
carbon nanotubes, allowed better osteogenic differentiation of
pre-osteoblasts. This was certified by elevated ALP activity and
the secretion of collagen type I [52].(Table 1)
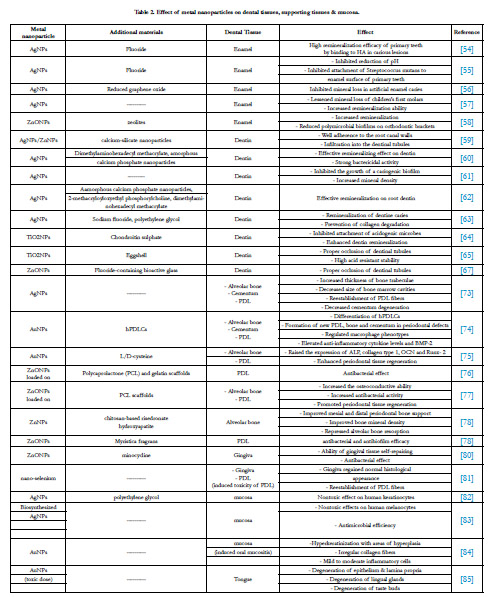
Dental and Supporting Tissue Regeneration
In recent years, dental tissue regeneration has gradually drawn
great attention [53].(Table 2)
Enamel regeneration
Nano silver fluoride (NSF) and AgNPs couldbind to HA crystals
in carious lesions and inhibited the reduction of pH and attachment
of Streptococcus mutans to the enamel surface leading to
remineralization of primary teeth [54, 55]. Reduced graphene oxide-
nanosilver could prevent mineral loss after biofilm challenge
[56]. They also lessen the mineral loss of children’s first molars
when incorporated with dental sealants [57].
Another investigation reported that zeolite-zinc oxide nanoparticles
showed high remineralization efficacy on enamel surface
against polymicrobial biofilms induced enamel lesions [58].
Dentin regeneration
Nanosilver and/or nanozinc incorporated with calcium-silicate
nanoparticles showed well adherence to the root canal walls and
infiltration into the dentinal tubules, suggesting their use as a root
canal disinfectant [59]. Meanwhile, an endodontic sealer embedded
with AgNPs displayed effective remineralizing and strengthening
influence on dentin [60].
AgNPs was able to produce silver chloride in dentin elevating
its mineral density [61]. In addition, bioactive multifunctional
composite containing AgNPs induced effective remineralization
by minerals precipitated on root dentin surfaces [62]. Moreover,
demineralized dentine blocks were subjected to a topical application
of sodium fluoride with polyethylene glycol (PEG)-AgNPs.
The mixture could remineralize dentine caries and prevent collagen
degradation [63].
On the other hand,Sereda et al. (2016) [64] revealed that titanium
oxide nanoparticles (TiO2NPs)with chondroitin sulphate inhibited
the attachment of acidogenic microbes which cause caries and
enhanced dentin remineralization. Onwubu et al. (2019) [65] in
another report documented thatTiO2NPs allow proper dentine
occlusion resulting incoverage of wide area of dentine.
It is considerable to mention that ZnO powder is added as a preservative
to toothpastes. The suspension hinders dentin demineralization
and shows antimicrobial property by releasing zinc ions
and ROS [66]. Furthermore, toothpastes having ZnONPs can
obturate dentinal tubules exposed to citric acid [67].
Nanomaterials offer substitutionalapproaches for dentin regeneration
by inducing odontogenic differentiation of human dental
pulp stem cells (DPSCs) [53]. DPSCs incorporated within IONPs
containing scaffolds could be differentiated into odontoblast-like
cells expressing dentin sialophosphoprotein and dentin matrix
protein 1 [68].
Pulp tissue regeneration
Pulp regeneration depends on revascularization at the root apex
[69]. Nano sized iron oxide -labeled SF/HA composite scaffold
was found to be good enhancerfor regeneration of DPSCs [68].
Nano scaffolds and nano drug deliver approacheslead the homing
of stem cells to realize dental pulp regeneration [70, 71].
Periodontium regeneration
Recent studies revealed promising results in the regeneration of
the periodontal apparatus [72]. Light microscopic examination of
old age rats’ periodontium receiving an oral dose of AgNPs demonstrated
increased thickness of bone trabeculae with decrease in
size of bone marrow cavities. Reestablishment of oblique, interradicular
and apical groups of periodontal ligament fibers (PDL)
were also observed. Nearly smooth surface of cementum with
amelioration of cementum degeneration and hypercementosis
was noticed [73].
Treatment with AuNPs in periodontitis inducedthe formation of
new periodontal attachment, bone and cementum in periodontal
defects, and reduced tissue destruction. Also, AuNPs could modify
the differentiation of hPDLCs, and control the early inflammatory
response by regulation of macrophage phenotypes andelevation
of anti-inflammatory cytokine levels and BMP-2 [74].
However, in a recent study, L/D-cysteineanchored AuNPs could
raise the expression of ALP, collagen type 1, OCN and Runx- 2
with promising effect on periodontal tissue regeneration [75].
Regarding ZnNPs, polycaprolactone (PCL) scaffolds loaded
with ZnONPs were utilized in periodontal regeneration and exhibited
antibacterial effect [76] and increased the osteoconductive
ability [77]. As well, local administration of chitosan-based
risedronate/zinc-hydroxyapatite nanoparticlesin dental pockets
showedmarked improvement in the mesial and distal periodontal
bone support, bone mineral density, and repressed alveolar bone
resorption [78].
Besides, combination of ZnONPs withMyristica fragrans could have antibacterial and antibiofilm efficacy, which has major role
in periodontitis [79]. Additionally, a serum albumin containing
ZnONPs showed high physicochemical properties and superior
antibacterial action for gingival tissue repair [80].
The protective effect of nano-selenium was studied on induced
aflatoxin B1 toxicity on PDL of rats. Jaw specimens presented
reestablishment and condensation of collagen fibers with normal
appearance of fibroblasts. Furthermore, gingiva regained its
slender, long and irregular epithelial ridges with intact basement
membrane. The lamina propria showed declinein collagen fiber
degeneration with reduction of the inflammatory cells. Surface
epithelium and lamina propria presented weak to moderate positive
immunostaining for caspase 3 [81].
Oral Mucosa and Salivary Glands
It was reported that AgNPs are safe for human keratinocytes and
exhibited high antimicrobial efficiencyfor concentrations up to 6
µg/mL [82, 83].
Nevertheless, AuNPs improved 5-florouracil induced oral mucositis
in hamsters and improved the parameters of inflammation
and oxidative stress. Re-epithelialization and hyperkeratinization
with areas of hyperplasia were noticed. The lamina propria presented
irregular collagen fibers with fibroblasts and mild to moderate
inflammatory cell infiltrate [84]. On contrary, examination
of the tongue of rats exposed to toxic over dose of AuNPs solution
revealed atrophic changes. Degenerationincluded the surface
epithelium and lamina propria of the dorsal and ventral surfaces
as well as the lingual salivary glands. The taste buds were degenerated
with destruction of their cells [85].(Table 2)
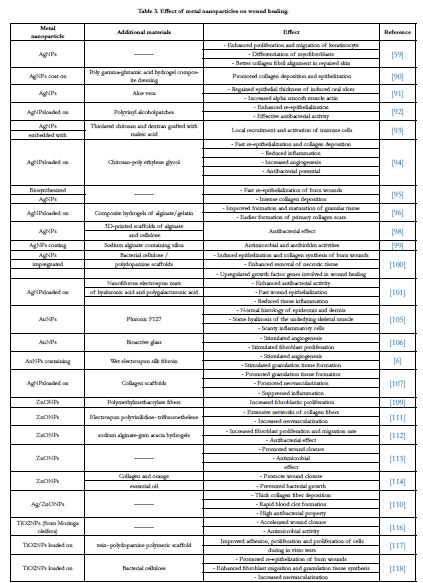
Wound Healing
MNPs are increasingly being used in skin and mucosal wounds, as
they speed up wound healing process andinhibit bacterial infections.
Moreover, they are easily to be used with lessened frequency
of dressing changes [86].(Table 3)
Silver nanoparticles (AgNPs)
AgNPshave significant role in wound dressings [87, 88]. They induce wound closure by enhancing the proliferation and migration of keratinocyte as well as differentiation of myofibroblasts with better collagen fibril alignment in repaired skin [89]. AgNPs poly gamma-glutamic acid hydrogel copolymer promoted collagen deposition and epithelization during healing of mouse wound in vivo [90]. Furthermore, application of Aloe vera and AgNPs on induced oral ulcers in mice showed regained epithelial thickness and upgraded alpha smooth muscle actin [91].
Composite hydrogels loaded with AgNPs accelerated the healing process of normal and diabetic ulcers by enhancing epithelial formation, collagen deposition and modulation of the host immune response [92-96]. It is supposed that the slow and continued release of metallic ions is required for wound healing applications [97].
Additionally, Bergonzi et al, (2020) [98] manufactured 3D-printed scaffolds of alginate and cellulose combined with AgNPs. The nanocomposites showed significant antibacterial effect. Same results were obtained with the application of sodium alginate containing silica coated AgNPs [99] Bacterial cellulose/polydopamine scaffolds embedded AgNPsalso showed enhanced removal of necrotic tissue, induced collagen synthesis and epithelization, andincreased expression of growth factor genes involved in wound healing [100]. Similarly, nanofibrous electrospun mats of hyaluronic acid and polygalacturonic acid loaded with nanosilver revealed antibacterial activity and wound epithelization [101]. It is worthy to mention that chronic wounds and burns are highly susceptible to infection. Therefore, existence of antimicrobial agents such as AgNPs in the scaffold design for wound healing is mandatory [6, 102].
Gold nanoparticles (AuNPs)
AuNPs have good biocompatibility and multifunctionality that aid the wound healing process [103]. It was demonstrated that AuNPs can damage the bacterial cell wall and bind to its DNA. Also, they may support the healing process by acting as antioxidants [104].
Burn-induced wounds in mice treated with AuNP-containing thermoresponsive gels revealed undamaged normal histology of epidermis and dermis with some hyalinosis of the underlying skeletal muscle and scanty inflammatory cells [105]. Marza and Magyari (2019) [106] and Fathi et al., (2019) [6] study results showed that bioactive glass combined with AuNPs, and AuNPs containing wet electrospun SF were able to stimulate angiogenesis and granulation tissue formation. Furthermore, AuNPs incorporated collagen scaffolds exhibited higher wound healing ability compared to pristine collagen scaffolds [107].
Zinc nanoparticles
ZnONPs are powerful antibacterial agents as they attack the bacterial cell membrane causing perforations [108]. Wound dressings containing these particles showincreased keratinocyte migration and rapid epithelialization [104]. Increased ?broblastic proliferation resulted when ZnONPs werecombined with polymethylmethacrylate ?bers [109]. Another research demonstrated that Ag/ZnONPs loaded with CS accelerated wound healing process in mice. The dressings showed rapid blood clot formation, thick collagen fiber deposition and high antibacterial property [110].
Identical results were documented with the use of electrospun polyvinilidine - trifluoroethelene zinc oxide nanocomposites [111] or nanoconjugates containing ZnONPs and sodium alginate-gum acacia hydrogels [112]. Added to this, Gao et al. (2017) [113] and- Balaure et al. (2018) [114] described dressings having ZnONPs which promote wound closure, hinder bacterial growth and show superior biocompatibility.
Titanium dioxide nanoparticles (TiO2NPs)
TiO2NPs were verified toincrease ROS production, which make them beneficial in wound management [115]. Sivaranjani et al. (2016) [116] synthesized TiO2NPs from Moringa oleifera leaves under certain conditions. They displayed enhanced wound healing and antimicrobial activities against gram-negative and gram-positive bacteria. Furthermore, TiO2NPs containing sca olds revealed good adhesion, and prolife ration of cells during in vitro tests [117]. Bacterial celluloseimpregnated TiO2NPs induced epithelial and granulation tissue synthesis, ?broblast migration and neovascularization in burns of mice models [118].
Conclusion
MNPs can be manufactured and improved with several chemical
functional groups, thus allowing them to be combined with various
ligands and drugs. This permits opening wide ranging uses in
biotechnology, which is credited to their unique physicochemical
properties.
In this overview, we have reviewed the possible beneficial applications
of MNPs in hard and soft tissues regeneration and in wound healing. It can be emphasized that metal nanoparticle-based materials
have favorable features for enhancing tissue regeneration,
hard tissue remineralization and stimulating wound healing. This
may point out a reference to investigators who are concerned in
metal nanomaterials biomedical uses, and offer high value of significance
as upcoming treatment modalities.
References
-
[1]. Gherasim O, Puiu RA, Bîrca AC, Burdu?el AC, Grumezescu AM. An Updated
Review on Silver Nanoparticles in Biomedicine. Nanomaterials (Basel).
2020 Nov 23;10(11):2318. PubMed PMID: 33238486.
[2]. Zurina IM, Presniakova VS, Butnaru DV, Svistunov AA, Timashev PS, Rochev YA. Tissue engineering using a combined cell sheet technology and scaffolding approach. Acta Biomater. 2020 Sep 1;113:63-83. PubMed PMID: 32561471.
[3]. Abbasian M, Massoumi B, Mohammad-Rezaei R, Samadian H, Jaymand M. Scaffolding polymeric biomaterials: Are naturally occurring biological macromolecules more appropriate for tissue engineering? Int J Biol Macromol. 2019 Aug 1;134:673-694. PubMed PMID: 31054302.
[4]. Nguyen MA, Camci-Unal G. Unconventional Tissue Engineering Materials in Disguise. Trends Biotechnol. 2020 Feb;38(2):178-190. PubMed PMID: 31590907.
[5]. Hasan A, Morshed M, Memic A, Hassan S, Webster TJ, Marei HE. Nanoparticles in tissue engineering: applications, challenges and prospects. Int J Nanomedicine. 2018 Sep 24;13:5637-5655. PubMed PMID: 30288038.
[6]. Fathi-Achachelouei M, Knopf-Marques H, Ribeiro da Silva CE, Barthès J, Bat E, Tezcaner A, et al. Use of Nanoparticles in Tissue Engineering and Regenerative Medicine. Front Bioeng Biotechnol. 2019 May 24;7:113. PubMed PMID: 31179276.
[7]. Vieira S, Vial S, Reis RL, Oliveira JM. Nanoparticles for bone tissue engineering. Biotechnol Prog. 2017 May;33(3):590-611. PubMed PMID: 28371447.
[8]. El-Sayed MA. Some interesting properties of metals confined in time and nanometer space of different shapes. Acc Chem Res. 2001 Apr;34(4):257- 64. PubMed PMID: 11308299.
[9]. Dos Santos Ramos MA, Da Silva PB, Spósito L, De Toledo LG, Bonifácio BV, Rodero CF, et al. Nanotechnology-based drug delivery systems for control of microbial biofilms: a review. Int J Nanomedicine. 2018 Feb 27;13:1179-1213. PubMed PMID: 29520143.
[10]. Iravani S. Metal Nanoparticles: Synthesis and Applications in Pharmaceutical Sciences.(1st ed) Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, , Ch. 2018; 2:15-31.
[11]. Li J, Li JJ, Zhang J, Wang X, Kawazoe N, Chen G. Gold nanoparticle size and shape influence on osteogenesis of mesenchymal stem cells. Nanoscale. 2016 Apr 21;8(15):7992-8007. PubMed PMID: 27010117.
[12]. Choi SY, Song MS, Ryu PD, Lam AT, Joo SW, Lee SY. Gold nanoparticles promote osteogenic differentiation in human adipose-derived mesenchymal stem cells through the Wnt/ß-catenin signaling pathway. Int J Nanomedicine. 2015 Jul 7;10:4383-92. PubMed PMID: 26185441.
[13]. Dayem AA, Choi HY, Yang GM, Kim K, Saha SK, Kim JH, et al. The potential of nanoparticles in stem cell differentiation and further therapeutic applications. Biotechnol J. 2016 Dec;11(12):1550-1560. PubMed PMID: 27797150.
[14]. Pan T, Song W, Gao H, Li T, Cao X, Zhong S, et al. miR-29b-Loaded Gold Nanoparticles Targeting to the Endoplasmic Reticulum for Synergistic Promotion of Osteogenic Differentiation. ACS Appl Mater Interfaces. 2016 Aug 3;8(30):19217-27. PubMed PMID: 27399270.
[15]. Li J, Zhang J, Chen Y, Kawazoe N, Chen G. TEMPO-Conjugated Gold Nanoparticles for Reactive Oxygen Species Scavenging and Regulation of Stem Cell Differentiation. ACS Appl Mater Interfaces. 2017 Oct 18;9(41):35683-35692. PubMed PMID: 28944661.
[16]. Yu M, Lei B, Gao C, Yan J, Ma PX. Optimizing surface-engineered ultrasmall gold nanoparticles for highly efficient miRNA delivery to enhance osteogenic differentiation of bone mesenchymal stromal cells. Nano Research. 2017 Jan;10(1):49-63.
[17]. Encabo-Berzosa MDM, Sancho-Albero M, Crespo A, Andreu V, Sebastian V, Irusta S, et al. The effect of PEGylated hollow gold nanoparticles on stem cell migration: potential application in tissue regeneration. Nanoscale. 2017 Jul 20;9(28):9848-9858. PubMed PMID: 28650026.
[18]. Zhang Y, Wang P, Wang Y, Li J, Qiao D, Chen R, et al. Gold Nanoparticles Promote the Bone Regeneration of Periodontal Ligament Stem Cell Sheets Through Activation of Autophagy. Int J Nanomedicine. 2021 Jan 6;16:61- 73. PubMed PMID: 33442250.
[19]. Eivazzadeh-Keihan R, Bahojb Noruzi E, Khanmohammadi Chenab K, Jafari A, Radinekiyan F, Hashemi SM, et al. Metal-based nanoparticles for bone tissue engineering. J Tissue Eng Regen Med. 2020 Dec;14(12):1687- 1714. PubMed PMID: 32914573.
[20]. Jung SK, Kim JH, Kim HJ, Ji YH, Kim JH, Son SW. Silver nanoparticleinduced hMSC proliferation is associated with HIF-1a-mediated upregulation of IL-8 expression. J Invest Dermatol. 2014 Dec;134(12):3003-3007. PubMed PMID: 24999595.
[21]. Zhang R, Lee P, Lui VC, Chen Y, Liu X, Lok CN, et al. Silver nanoparticles promote osteogenesis of mesenchymal stem cells and improve bone fracture healing in osteogenesis mechanism mouse model. Nanomedicine. 2015 Nov;11(8):1949-59. PubMed PMID: 26282383.
[22]. Kumar Saini R, Prasad Bagri L, Bajpai AK. Nano-silver hydroxyapatite based antibacterial 3D scaffolds of gelatin/alginate/poly (vinyl alcohol) for bone tissue engineering applications. Colloids Surf B Biointerfaces. 2019 May 1;177:211-218. PubMed PMID: 30743068.
[23]. Paterson TE, Shi R, Tian J, Harrison CJ, De Sousa Mendes M, Hatton PV, et al. Electrospun Scaffolds Containing Silver-Doped Hydroxyapatite with Antimicrobial Properties for Applications in Orthopedic and Dental Bone Surgery. J Funct Biomater. 2020 Aug 14;11(3):58. PubMed PMID: 32824017.
[24]. Zhang C, Lan J, Wang S, Han S, Yang H, et al. Silver nanowires on acidalkali- treated titanium surface: Bacterial attachment and osteogenic activity. Ceram Int. 2019 Dec 15;45: 24528-24537.
[25]. Rafieerad A, Bushroa A, Nasiri-Tabrizi B, Baradaran S, Amiri A, et al. Simultaneous enhanced antibacterial and osteoblast cytocompatibility performance of Ti6Al7Nb implant by nano-silver/graphene oxide decorated mixed oxide nanotube composite. Surf Coat Technol. 2019 Feb 25;360:181-195.
[26]. Zeng X, Xiong S, Zhuo S, Liu C, Miao J, Liu D, et al. Nanosilver/poly (dllactic- co-glycolic acid) on titanium implant surfaces for the enhancement of antibacterial properties and osteoinductivity. Int J Nanomedicine. 2019 Mar 11;14:1849-1863. PubMed PMID: 30880984.
[27]. MareciD, Trinca L, Cailean D, Souto R. Corrosion resistance of ZrTi alloys with hydroxyapatite zirconia-silver layer in simulated physiological solution containing proteins for biomaterial applications. Appl Surf Sci. 2016 Dec 15;389:1069-1075.
[28]. Trinca L, Mareci D, Souto R, Lozano-Gorrín A, Izquierdo J, et al. Osseointegration evaluation of ZrTi alloys with hydroxyapatite-zirconia-silver layer in pig’s tibiae. Appl Surf Sci. 2019 Sep 1;487:127-137.
[29]. Bakhsheshi-Rad HR, Ismail AF, Aziz M, Akbari M, Hadisi Z, Khoshnava SM, et al. Co-incorporation of graphene oxide/silver nanoparticle into poly- L-lactic acid fibrous: A route toward the development of cytocompatible and antibacterial coating layer on magnesium implants. Mater Sci Eng C Mater Biol Appl. 2020 Jun;111:110812. PubMed PMID: 32279830.
[30]. Abdelaziz D, Hefnawy A, Al-Wakeel E, El-Fallal A, El-Sherbiny IM. New biodegradable nanoparticles-in-nanofibers based membranes for guided periodontal tissue and bone regeneration with enhanced antibacterial activity. J Adv Res. 2020 Jun 20;28:51-62. PubMed PMID: 33364045.
[31]. Kumar P, Dehiya B, Sindhu A, Kumar R, Pruncu C, et al. Fabrication and characterization of silver nanorods incorporated calcium silicate scaffold using polymeric sponge replica technique. Mater Des. 2020 Oct 1;195:109026.
[32]. Wenhao Z, Zhang T, Yan J, Li Q, Xiong P, Li Y, et al. In vitro and in vivo evaluation of structurally-controlled silk fibroin coatings for orthopedic infection and in-situ osteogenesis. Acta Biomater. 2020 Oct 15;116:223-245. PubMed PMID: 32889111.
[33]. Wang Q, Chen B, Ma F, Lin S, Cao M, et al. Magnetic iron oxide nanoparticles accelerate osteogenic differentiation of mesenchymal stem cells via modulation of long noncoding RNA INZEB2. Biomaterials. 2017;10(2):626-642.
[34]. Jiang P, Zhang Y, Zhu C, Zhang W, Mao Z, Gao C. Fe3O4/BSA particles induce osteogenic differentiation of mesenchymal stem cells under static magnetic field. Acta Biomater. 2016 Dec;46:141-150. PubMed PMID: 27646502.
[35]. Levy I, Sher I, Corem-Salkmon E, Ziv-Polat O, Meir A, Treves AJ, et al. Bioactive magnetic near Infra-Red fluorescent core-shell iron oxide/human serum albumin nanoparticles for controlled release of growth factors for augmentation of human mesenchymal stem cell growth and differentiation. J Nanobiotechnology. 2015 May 7;13:34. PubMed PMID: 25947109.
[36]. Chen Z, Ni S, Han S, Crawford R, Lu S, Wei F, Chang J, Wu C, Xiao Y. Nanoporous microstructures mediate osteogenesis by modulating the osteoimmune response of macrophages. Nanoscale. 2017 Jan 5;9(2):706-718. PubMed PMID: 27959374.
[37]. Glenske K, Donkiewicz P, Köwitsch A, Milosevic-Oljaca N, Rider P, Rofall S, et al. Applications of Metals for Bone Regeneration. Int J Mol Sci. 2018 Mar 12;19(3):826. PubMed PMID: 29534546.
[38]. Dhivya S, Ajita J, Selvamurugan N. Metallic Nanomaterials for Bone Tissue Engineering. J Biomed Nanotechnol. 2015 Oct;11(10):1675-700. PubMed PMID: 26502634.
[39]. Forero JC, Roa E, Reyes JG, Acevedo C, Osses N. Development of Useful Biomaterial for Bone Tissue Engineering by Incorporating Nano-Copper- Zinc Alloy (nCuZn) in Chitosan/Gelatin/Nano-Hydroxyapatite (Ch/G/ nHAp) Scaffold. Materials (Basel). 2017 Oct 17;10(10):1177. PubMed PMID: 29039747.
[40]. Hejazy M, Koohi M, Pour, B, Najafi D. Toxicity of manufactured copper nanoparticles—A review. Nanomedicine Research Journal. 2018 Jan 1;3:1- 9.
[41]. aidev LR, Kumar S, Chatterjee K. Multi-biofunctional polymer graphene composite for bone tissue regeneration that elutes copper ions to impart angiogenic, osteogenic and bactericidal properties. Colloids Surf B Biointerfaces. 2017 Nov 1;159:293-302. PubMed PMID: 28802737.
[42]. Sahmani S, Shahali M, Nejad M, Khandan A, Aghdam M, et al. Effect of copper oxide nanoparticles on electrical conductivity and cell viability of calcium phosphate scaffolds with improved mechanical strength for bone tissue engineering. The European Physical Journal Plus. 2019 Jan;134:7.
[43]. Oshima Y, Iwasa F, Tachi K, Baba K. Effect of Nanofeatured Topography on Ceria-Stabilized Zirconia/Alumina Nanocomposite on Osteogenesis and Osseointegration. Int J Oral Maxillofac Implants. 2017 Jan/Feb;32(1):81- 91. PubMed PMID: 28095516.
[44]. Smieszek A, Szydlarska J, Mucha A, Chrapiec M, Marycz K. Enhanced cytocompatibility and osteoinductive properties of sol-gel-derived silica/zirconium dioxide coatings by metformin functionalization. J Biomater Appl. 2017 Nov;32(5):570-586. PubMed PMID: 29113566.
[45]. Bhowmick A, Pramanik N, Jana P, Mitra T, Gnanamani A, Das M, Kundu PP. Development of bone-like zirconium oxide nanoceramic modified chitosan based porous nanocomposites for biomedical application. Int J Biol Macromol. 2017 Feb;95:348-356. PubMed PMID: 27865958.
[46]. Doostmohammadi A, Karimzadeh Esfahani Z, Ardeshirylajimi A, Rahmati Dehkordi Z. Zirconium modified calcium-silicate- based nanoceramics: An in vivo evaluation in a rabbit tibial defect model. International Journal of Applied Ceramic Technology. 2019 Mar;16:431-437.
[47]. Maghsoudlou MA, Nassireslami E, Saber-Samandari S, Khandan A. Bone Regeneration Using Bio-Nanocomposite Tissue Reinforced with Bioactive Nanoparticles for Femoral Defect Applications in Medicine. Avicenna J Med Biotechnol. 2020 Apr-Jun;12(2):68-76. PubMed PMID: 32431790.
[48]. Li K, Shen Q, Xie Y, You M, Huang L, Zheng X. Incorporation of cerium oxide into hydroxyapatite coating regulates osteogenic activity of mesenchymal stem cell and macrophage polarization. J Biomater Appl. 2017 Feb;31(7):1062-1076. PubMed PMID: 27932702.
[49]. Xiang J, Li J, He J, Tang X, Dou C, Cao Z, et al. Cerium Oxide Nanoparticle Modified Scaffold Interface Enhances Vascularization of Bone Grafts by Activating Calcium Channel of Mesenchymal Stem Cells. ACS Appl Mater Interfaces. 2016 Feb;8(7):4489-99. PubMed PMID: 26824825.
[50]. Li J, Wen J, Li B, Li W, Qiao W, Shen J, et al. Valence State Manipulation of Cerium Oxide Nanoparticles on a Titanium Surface for Modulating Cell Fate and Bone Formation. Adv Sci (Weinh). 2017 Dec 18;5(2):1700678. PubMed PMID: 29610729.
[51]. Laurenti M, Cauda V. ZnO Nanostructures for Tissue Engineering Applications. Nanomaterials (Basel). 2017 Nov 6;7(11):374. PubMed PMID: 29113133.
[52]. Shrestha B, Shrestha S, Tiwari A, Kim J, Ko S, et al. Bio-inspired hybrid scaffold of zinc oxide-functionalized multi-wall carbon nanotubes reinforced polyurethane nanofibers for bone tissue engineering. Mater Des. 2017 Nov 5;133:69-81.
[53]. Ding Q, Cui J, Shen H, He C, Wang X, Shen SGF, Lin K. Advances of nanomaterial applications in oral and maxillofacial tissue regeneration and disease treatment. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020 Oct 8:e1669. PubMed PMID: 33090719.
[54]. Nozari A, Ajami S, Rafiei A, Niazi E. Impact of Nano Hydroxyapatite, Nano Silver Fluoride and Sodium Fluoride Varnish on Primary Teeth Enamel Remineralization: An In Vitro Study. J Clin Diagn Res. 2017 Sep;11(9):ZC97-ZC100. PubMed PMID: 29207844.
[55]. Silva A, Teixeira J, Mota C, Lins E, Júnior P, et al. In Vitro morphological, optical and microbiological evaluation of nanosilver fluoride in the remineralization of deciduous teeth enamel. Nanotechnol Rev. 2018 Dec 1;7(6):509-520.
[56]. Wu R, Zhao Q, Lu S, Fu Y, Yu D, Zhao W. Inhibitory effect of reduced graphene oxide-silver nanocomposite on progression of artificial enamel caries. J Appl Oral Sci. 2018 Dec 10;27:e20180042. PubMed PMID: 30540069.
[57]. Salas-López EK, Pierdant-Pérez M, Hernández-Sierra JF, Ruíz F, Mandeville P, Pozos-Guillén AJ. Effect of Silver Nanoparticle-Added Pit and Fissure Sealant in the Prevention of Dental Caries in Children. J Clin Pediatr Dent. 2017;41(1):48-52. PubMed PMID: 28052214.
[58]. Pourhajibagher M, Bahador A. Enhanced reduction of polymicrobial biofilms on the orthodontic brackets and enamel surface remineralization using zeolite-zinc oxide nanoparticles-based antimicrobial photodynamic therapy. BMC Microbiol. 2021 Oct 7;21(1):273. PubMed PMID: 34620084.
[59]. Zhu J, Liang R, Sun C, Xie L, Wang J, Leng D, et al. Effects of nanosilver and nanozinc incorporated mesoporous calcium-silicate nanoparticles on the mechanical properties of dentin. PLoS One. 2017 Aug 7;12(8):e0182583. PubMed PMID: 28787004.
[60]. Baras BH, Sun J, Melo MAS, Tay FR, Oates TW, Zhang K, et al. Novel root canal sealer with dimethylaminohexadecyl methacrylate, nano-silver and nano-calcium phosphate to kill bacteria inside root dentin and increase dentin hardness. Dent Mater. 2019 Oct;35(10):1479-1489. PubMed PMID: 31387742.
[61]. Yin IX, Yu OY, Zhao IS, Mei ML, Li QL, Tang J, et al. Developing biocompatible silver nanoparticles using epigallocatechin gallate for dental use. Arch Oral Biol. 2019 Jun;102:106-112. PubMed PMID: 30999064.
[62]. Xiao S, Liang K, Weir MD, Cheng L, Liu H, Zhou X, et al. Combining Bioactive Multifunctional Dental Composite with PAMAM for Root Dentin Remineralization. Materials (Basel). 2017 Jan 22;10(1):89. PubMed PMID: 28772450.
[63]. Zhao IS, Yin IX, Mei ML, Lo ECM, Tang J, Li Q, et al. Remineralising Dentine Caries Using Sodium Fluoride with Silver Nanoparticles: An In Vitro Study. Int J Nanomedicine. 2020 Apr 23;15:2829-2839. PubMed PMID: 32368057.
[64]. Sereda G, Rashwan K, Karels B, Fritza A. Novel Materials for Desensitizing and Remineralizing Dentifrices. Advanced Materials Tech Connect Briefs. 2016;1:135-138.
[65]. Onwubu SC, Mdluli PS, Singh S, Tlapana T. A novel application of nano eggshell/titanium dioxide composite on occluding dentine tubules: an in vitro study. Braz Oral Res. 2019 Mar 18;33:e016. PubMed PMID: 30892411.
[66]. Bakri MM, Hossain MZ, Razak FA, Saqina ZH, Misroni AA, Ab-Murat N, et al. Dentinal tubules occluded by bioactive glass-containing toothpaste exhibit high resistance toward acidic soft drink challenge. Aust Dent J. 2017 Jun;62(2):186-191. PubMed PMID: 27813093.
[67]. Khan AS, Farooq I, Alakrawi KM, Khalid H, Saadi OW, Hakeem AS. Dentin Tubule Occlusion Potential of Novel Dentifrices Having Fluoride Containing Bioactive Glass and Zinc Oxide Nanoparticles. Med Princ Pract. 2020;29(4):338-346. PubMed PMID: 31698358.
[68]. Zhang W, Zheng Y, Liu H, Zhu X, Gu Y, Lan Y, et al. A non-invasive monitoring of USPIO labeled silk fibroin/hydroxyapatite scaffold loaded DPSCs for dental pulp regeneration. Mater Sci Eng C Mater Biol Appl. 2019 Oct;103:109736. PubMed PMID: 31349524.
[69]. Li X, Ma C, Xie X, Sun H, Liu X. Pulp regeneration in a full-length human tooth root using a hierarchical nanofibrous microsphere system. Acta Biomater. 2016 Apr 15;35:57-67. PubMed PMID: 26931056.
[70]. Bottino MC, Pankajakshan D, Nör JE. Advanced Scaffolds for Dental Pulp and Periodontal Regeneration. Dent Clin North Am. 2017 Oct;61(4):689- 711. PubMed PMID: 28886764.
[71]. Eramo S, Natali A, Pinna R, Milia E. Dental pulp regeneration via cell homing. Int Endod J. 2018 Apr;51(4):405-419. PubMed PMID: 29047120. [72]. Moradpoor H, Safaei M, Mozaffari H, Sharif R, Imani M, et al. An overview of recent progress in dental applications of zinc oxide nanoparticles. RSC Adv. 2021;11:21189-21206.
[73]. Haggag T, Mahmoud E. Histological study of reparative capacity of silver nanoparticles on age related changes of alveolar bone, cementum and periodontal ligament in rats. EDJ. 2018;64:3511-3521.
[74]. Ni C, Zhou J, Kong N, Bian T, Zhang Y, Huang X, Xiao Y, Yang W, Yan F. Gold nanoparticles modulate the crosstalk between macrophages and periodontal ligament cells for periodontitis treatment. Biomaterials. 2019 Jun;206:115-132. PubMed PMID: 30933774.
[75]. Zhang S, Zhou H, Kong N, Wang Z, Fu H, Zhang Y, et al. l-cysteinemodified chiral gold nanoparticles promote periodontal tissue regeneration. Bioact Mater. 2021 Mar 13;6(10):3288-3299. PubMed PMID: 33778205.
[76]. BottinoM. Nanofibers for Regenerative Dentistry: From Scaffolds to Drug Delivery Systems. Microsc Microanal. 2016 Jul;22:996-997.
[77]. Nasajpour A, Ansari S, Rinoldi C, Shahrokhi Rad A, Aghaloo T, et al. A Multifunctional Polymeric Periodontal Membrane with Osteogenic and Antibacterial Characteristics. Adv Funct Mater. 2018;28(3):1703437.
[78]. Khajuria DK, Zahra SF, Razdan R. Effect of locally administered novel biodegradable chitosan based risedronate/zinc-hydroxyapatite intra-pocket dental film on alveolar bone density in rat model of periodontitis. J Biomater Sci Polym Ed. 2018 Jan;29(1):74-91. PubMed PMID: 29088987.
[79]. Cherian T, Ali K, Fatima S, Saquib Q, Ansari SM, Alwathnani HA, Al- Khedhairy AA, et al. Myristica fragrans bio-active ester functionalized ZnO nanoparticles exhibit antibacterial and antibiofilm activities in clinical isolates. J Microbiol Methods. 2019 Nov;166:105716. PubMed PMID: 31499093.
[80]. Mou J, Liu Z, Liu J, Lu J, Zhu W, Pei D. Hydrogel containing minocycline and zinc oxide-loaded serum albumin nanopartical for periodontitis application: preparation, characterization and evaluation. Drug Deliv. 2019 Dec;26(1):179-187. PubMed PMID: 30822158.
[81]. Hamed G, Selim M. Protective effect of nano-selenium against experimentally induced toxicity by aflatoxin b1 (afb1) on the gingiva and periodontal ligament of albino rats (histological and immunohistochemical study). EDJ. 2021;67:357-366.
[82]. Pinzaru I, Coricovac D, Dehelean C, Moaca EA, Mioc M, Baderca F, et al. Stable PEG-coated silver nanoparticles - A comprehensive toxicological profile. Food Chem Toxicol. 2018 Jan;111:546-556. PubMed PMID: 29191727.
[83]. Khalil NM, Abd El-Ghany MN, Rodríguez-Couto S. Antifungal and antimycotoxin efficacy of biogenic silver nanoparticles produced by Fusarium chlamydosporum and Penicillium chrysogenum at non-cytotoxic doses. Chemosphere. 2019 Mar;218:477-486. PubMed PMID: 30497030.
[84]. Vilar CJF, Ribeiro SB, de Araújo AA, Guerra GCB, de Araújo Júnior RF, Brito GAC, et al. Effect of Gold Nanoparticle on 5-Fluorouracil-Induced Experimental Oral Mucositis in Hamsters. Pharmaceutics. 2020 Mar 27;12(4):304. PubMed PMID: 32230975.
[85]. El-Halwagy M, Hegazy E, Youssef M, Ghali L. The potential impacts of gold nanoparticles on the mucous membrane of the albino rats’ tongues. Dental science updates. 2020 Mar 1;1:63-73.
[86]. Mihai MM, Dima MB, Dima B, Holban AM. Nanomaterials for Wound Healing and Infection Control. Materials (Basel). 2019 Jul 6;12(13):2176. PubMed PMID: 31284587.
[87]. Mehrabani MG, Karimian R, Mehramouz B, Rahimi M, Kafil HS. Preparation of biocompatible and biodegradable silk fibroin/chitin/silver nanoparticles 3D scaffolds as a bandage for antimicrobial wound dressing. Int J Biol Macromol. 2018 Jul 15;114:961-971. PubMed PMID: 29581004.https:// pubmed.ncbi.nlm.nih.gov/29581004/
[88]. Rahimi M, Ahmadi R, Samadi Kafil H, Shafiei-Irannejad V. A novel bioactive quaternized chitosan and its silver-containing nanocomposites as a potent antimicrobial wound dressing: Structural and biological properties. Mater Sci Eng C Mater Biol Appl. 2019 Aug;101:360-369. PubMed PMID: 31029329.
[89]. Kwan KH, Liu X, To MK, Yeung KW, Ho CM, Wong KK. Modulation of collagen alignment by silver nanoparticles results in better mechanical properties in wound healing. Nanomedicine. 2011 Aug;7(4):497-504. PubMed PMID: 21272666.
[90]. Wang Y, Dou C, He G, Ban L, Huang L, Li Z, et al. Biomedical Potential of Ultrafine Ag Nanoparticles Coated on Poly (Gamma-Glutamic Acid) Hydrogel with Special Reference to Wound Healing. Nanomaterials (Basel). 2018 May 14;8(5):324. PubMed PMID: 29757942.
[91]. El-Batal AI, Ahmed SF. Therapeutic effect of Aloe vera and silver nanoparticles on acid-induced oral ulcer in gamma-irradiated mice. Braz Oral Res. 2018 Feb 5;32:e004. PubMed PMID: 29412224.
[92]. Ahsan A, Farooq M. Therapeutic potential of green synthesized silver nanoparticles loaded PVA hydrogel patches for wound healing. J Drug Deliv Sci Technol. 2019 Dec 1;54:101308.
[93]. Shi G, Chen W, Zhang Y, Dai X, Zhang X, Wu Z. An Antifouling Hydrogel Containing Silver Nanoparticles for Modulating the Therapeutic Immune Response in Chronic Wound Healing. Langmuir. 2019 Feb 5;35(5):1837- 1845. PubMed PMID: 30086636.
[94]. Masood N, Ahmed R, Tariq M, Ahmed Z, Masoud MS, Ali I, et al. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int J Pharm. 2019 Mar 25;559:23-36. PubMed PMID: 30668991.
[95]. Ying W, Tan J, Chen C, Sun T, Wang S, et al. Biofabrication of silver nanoparticles and its application for development of wound dressing system in nursing care for burn injuries in children. J Drug Deliv Sci Technol. 2019 Dec 1;54:101236.
[96]. Diniz FR, Maia RCAP, Rannier L, Andrade LN, V Chaud M, da Silva CF, et al. Silver Nanoparticles-Composing Alginate/Gelatine Hydrogel Improves Wound Healing In Vivo. Nanomaterials (Basel). 2020 Feb 23;10(2):390. PubMed PMID: 32102229.
[97]. Nešovi´c K, Jankovi´c A, Radeti´c T, Vukašinovi´c-Sekuli´c M, Koji´c V, et al. Chitosan-based hydrogel wound dressings with electrochemically incorporated silver nanoparticles—In vitro study. Eur Polym J. 2019 Dec 1;121:109257.
[98]. Bergonzi C, Remaggi G, Graiff C, Bergamonti L, Potenza M, Ossiprandi MC, et al. Three-Dimensional (3D) Printed Silver Nanoparticles/Alginate/ Nanocrystalline Cellulose Hydrogels: Study of the Antimicrobial and Cytotoxicity Efficacy. Nanomaterials (Basel). 2020 Apr 28;10(5):844. PubMed PMID: 32353965.
[99]. Ambrogi V, Pietrella D, Donnadio A, Latterini L, Di Michele A, Luffarelli I, et al. Biocompatible alginate silica supported silver nanoparticles composite films for wound dressing with antibiofilm activity. Mater Sci Eng C Mater Biol Appl. 2020 Jul;112:110863. PubMed PMID: 32409034.
[100]. Jiji S, Udhayakumar S, Maharajan K, Rose C, Muralidharan C, Kadirvelu K. Bacterial cellulose matrix with in situ impregnation of silver nanoparticles via catecholic redox chemistry for third degree burn wound healing. Carbohydr Polym. 2020 Oct 1;245:116573. PubMed PMID: 32718650.
[101]. El-Aassar MR, Ibrahim OM, Fouda MMG, El-Beheri NG, Agwa MM. Wound healing of nanofiber comprising Polygalacturonic/Hyaluronic acid embedded silver nanoparticles: In-vitro and in-vivo studies. Carbohydr Polym. 2020 Jun 15;238:116175. PubMed PMID: 32299548.
[102]. Yang J, Chen Y, Zhao L, Feng Z, Peng K, et al. Preparation of a chitosan/ carboxymethyl chitosan/AgNPs polyelectrolyte composite physical hydrogel with self-healing ability, antibacterial properties, and good biosafety simultaneously, and its application as a wound dressing. Compos Part B Eng. 2020 Sep 15;197:108139.
[103]. Berthet M, Gauthier Y, Lacroix C, Verrier B, Monge C. Nanoparticle- Based Dressing: The Future of Wound Treatment? Trends Biotechnol. 2017 Aug;35(8):770-784. PubMed PMID: 28645529.
[104]. Vijayakumar V, Samal SK, Mohanty S, Nayak SK. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int J Biol Macromol. 2019 Feb 1;122:137-148. PubMed PMID: 30342131.
[105]. Arafa MG, El-Kased RF, Elmazar MM. Thermoresponsive gels containing gold nanoparticles as smart antibacterial and wound healing agents. Sci Rep. 2018 Sep 12;8(1):13674. PubMed PMID: 30209256.
[106]. Mârza SM, Magyari K, Bogdan S, Moldovan M, Pestean C, et al. Skin wound regeneration with bioactive glass-gold nanoparticles ointment. Biomed Mater. 2019 Feb 8;14(2):025011. PubMed PMID: 30630137.
[107]. Nethi SK, Das S, Patra CR, Mukherjee S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater Sci. 2019 Jul 1;7(7):2652-2674. PubMed PMID: 31094374.
[108]. Hamdan S, Pastar I, Drakulich S, Dikici E, Tomic-Canic M, Deo S, et al. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Cent Sci. 2017 Mar 22;3(3):163-175. PubMed PMID: 28386594.
[109]. Balen R, de Costa W, de Lara Andrade J, Piai J, Muniz E, et al.Structural, thermal, optical properties and cytotoxicity of PMMA/ZnO fibers and films: Potential application in tissue engineering. Appl Surf Sci. 2016 Nov 1;385:257-267.
[110]. Lu Z, Gao J, He Q, Wu J, Liang D, Yang H, et al. Enhanced antibacterial and wound healing activities of microporous chitosan-Ag/ZnO composite dressing. Carbohydr Polym. 2017 Jan 20;156:460-469. PubMed PMID: 27842847.
[111]. Augustine R, Dan P, Sosnik A, Kalarikkal N, Tran N, et al.Electrospun poly(vinylidene fluoride-trifluoroethylene)/zinc oxide nanocomposite tissue engineering scaffolds with enhanced cell adhesion and blood vessel formation. Nano Res. 2017 Oct 1;10(10):3358-3376.
[112]. Raguvaran R, Manuja BK, Chopra M, Thakur R, Anand T, Kalia A, et al. Sodium alginate and gum acacia hydrogels of ZnO nanoparticles show wound healing effect on fibroblast cells. Int J Biol Macromol. 2017 Mar;96:185-191. PubMed PMID: 27939272.
[113]. Gao Y , Han Y , Cui M , Tey HL , Wang L , Xu C . ZnO nanoparticles as an antimicrobial tissue adhesive for skin wound closure. J Mater Chem B. 2017 Jun 21;5(23):4535-4541. PubMed PMID: 32263980.
[114]. Balaure PC, Holban AM, Grumezescu AM, Mogosanu GD, Balseanu TA, Stan MS, et al. In vitro and in vivo studies of novel fabricated bioactive dressings based on collagen and zinc oxide 3D scaffolds. Int J Pharm. 2019 Feb 25;557:199-207. PubMed PMID: 30597267.
[115]. Nikolova MP, Chavali MS. Metal Oxide Nanoparticles as Biomedical Materials. Biomimetics (Basel). 2020 Jun 8;5(2):27. PubMed PMID: 32521669.
[116]. Sivaranjani V, Philominathan P. Synthesize of Titanium dioxide nanoparticles using Moringa oleifera leaves and evaluation of wound healing activity. Wound Med. 2016 Mar 1;12:1-5.
[117]. Babitha S, Korrapati PS. Biodegradable zein-polydopamine polymeric scaffold impregnated with TiO2 nanoparticles for skin tissue engineering. Biomed Mater. 2017 Sep 25;12(5):055008. PubMed PMID: 28944761.
[118]. Khalid A, Ullah H, Ul-Islam M, Khan R Khan S, et al.Bacterial cellulose– TiO2 nanocomposites promote healing and tissue regeneration in burn mice model. RSC Adv. 2017;7:47662.