Effects of Probiotics on Candida albicans in Oral Cavity of Children with Leukemia during Chemotherapy
Nisa P Biantama1, Margaretha Suharsini2*, Mochammad Fahlevi Rizal3
1 Postgraduate Program, Department of Pediatric Dentistry, Faculty of Dentistry, Universitas Indonesia, Jakarta 10430, Indonesia.
2 Professor, Department of Pediatric Dentistry, Faculty of Dentistry, Universitas Indonesia, Jakarta 10430, Indonesia.
3 Lecturer, Department of Pediatric Dentistry, Faculty of Dentistry, Universitas Indonesia, Jakarta 10430, Indonesia.
*Corresponding Author
Prof. Dr.drg. Margaretha Suharsini Soetopo, SU., SpKGA(K),
Professor, Department of Pediatric Dentistry, Faculty of Dentistry, Universitas Indonesia, Jakarta 10430, Indonesia.
E-mail: m_suharsini@ui.ac.id
Received: December 29, 2021; Accepted: January 30, 2022; Published: February 25, 2022
Citation: Nisa P Biantama, Margaretha Suharsini, Mochammad Fahlevi Rizal. Effects of Probiotics on Candida albicans in Oral Cavity of Children with Leukemia during Chemotherapy. Int J Dentistry Oral Sci. 2022;9(2):5249-5253. doi: dx.doi.org/10.19070/2377-8075-220001053
Copyright: Margaretha Suharsini©2022. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: Candida albicans is the leading cause of opportunistic microbial infections in patients with cancer. Acute Lymphocytic
Leukemia patients undergoing chemotherapy have high risk of candidiasis due to immunosuppression and weakened
epithelial barriers. Systemic antifungal drugs’ usage is limited by the greater risk of side effects and developing resistant
strains, yet currently available topical antifungal drugs are still considered ineffective for immunosuppressed patients including
chemotherapy patients.Several studies provide evidence for the feasibility of probiotic Lactobacillus casei to act as alternative
antifungal in various human organ systems.
Objectives: To analyze the effects of probiotics on the number of C. albicans in oral cavity of children with leukemia during
chemotherapy.
Materials and Methods: Saliva samples were taken from 11 children with leukemia during chemotherapy. Subjects were
instructed to do mouth rinse with probiotics contained Lactobacillus casei for 60s, twice daily, over the course of 14 days.
Unstimulated saliva samples were collected sequentially at 3 time points (baseline, 7 days, and 14 days). The number of C.
albicans was quantified by qPCR.
Results: Statiscally significant differences were found between the number of C. albicans at baseline (494.363+180.737 CFU/
ml), after 7 days (276.653+69.903 CFU/ml), and after 14 days (229.286+50.883 CFU/ml) mouth rinsing with probiotics.
Significant lower number was found both after 7 and 14 days rinsing with probiotics (p<0.05).
Conclusion: Probiotics has reducing effects on the number of C. albicans in oral cavity of children with leukemia during
chemotherapy.
2.Introduction
3.Materials and Methods
3.Results
4.Discussion
5.Conclusion
5.References
Keywords
Probiotic; Candida albicans; Candidiasis; Leukemia; Chemotherapy.
Introduction
Cancer is a leading cause of death in children throughout the
world. About 300,000 children aged 0-19 years are diagnosed with
cancer each year with leukemia as the most common type [1, 2]
Chemotherapy is one of the basic and most widely used treatment
modalities for cancer. Paediatric hematologic cancer, specifically
acute lymphoblastic leukemia (ALL), was taken as terminal
disease several decades ago. Now, it is known that with a course
of combination chemotherapy, 75-80% of children with ALL are
cured.[3] Chemotherapy works by giving a group of drugs that
can inhibit growth or kill cancer cells. However, all chemotherapeutic
drugs are cytotoxic by nature not only to cancer cells but
also to normal cells. While damaging cancer cells, chemotherapy
also gives damage to cells that divide rapidly under normal circumstances,
such as digestive tract lining cells including oral epithelium.[
4, 5]
At present, oral mucositis is considered to be the most severeand
most common non-haematological side effect associated with
chemotherapy.[6, 7] Younger cancer patients who undergo chemotherapy
are more at risk of oral mucositis due to the high mitotic
rate of oral mucosal epithelial cells. Oral mucositis occur in up to
90% of chemotherapy patients under 12 years old.[7, 8] Destruction
of epithelial barriers, which are the host’s first line of defence
against microorganisms invasions, will increase the susceptibility for microbial infections to occur,including systemic microbial infections
that can be highly life-threatening in immunosuppressed
patients.[9] Candida albicansis the main cause of opportunistic microbial
infections in patients with cancer. Cancer patients have
high risk of fungal infections primarily due to the depression of
host immune cells and weakened epithelial barriers induced by
chemotherapy.[10]
In normal circumstances, Candida albicans coexists as a fungal
commensal with other microorganisms from normal oral flora
and does not cause infection. However, changes in the oral and/
or systemic environment can result in imbalance of this species
causing infection. Changes in the oral and systemic environment
of cancer patients undergoing chemotherapy such as disruption
to epithelial barriers and immunosuppression result in overgrowth
of Candida thereby facilitating fungal infection.[10] For decades,
systemic antifungal drugs have been used to prevent Candida infections.
However, due to drug side effects (nausea, vomiting, and
diarrhea) and the potential for emergence of resistant strains, systemic
antifungal has not been considered to be fully successful.
The toxicity and emergence of resistance from antifungal drugs
that are currently available are of concern in the health sector, and
thus alternative therapies are urgently needed.[11]
Probiotics are defined as living microorganisms, which when given
or consumed in sufficient quantities, provide health benefits to
the host.[12] Lactobacillus organisms have been used as probiotics
for decades and previous studies show evidence of Lactobacillus
strains’ feasibility to act as alternative biological approaches to
combating pathogenic fungi in various human organ systems, including
the oral cavity.[13-15] There is no study that shows effects
of probiotic on the number of Candida albicansin the oral cavity
of children with leukemia who are undergoing chemotherapy.
This study was considered as a preliminary study with the aim to
investigate the effects of probiotics on the quantitative levels of
Candida albicansin the oral cavity of children with leukemia during
chemotherapy.
Materials and Methods
This study was approved by the Dental Research Ethics Committee,
Faculty of Dentistry Universitas Indonesia. Clinical experimental
study was performed to evaluate the quantitative levels of
Candida albicansin the oral cavity through saliva samples.
The subjects were consisted of 11 children with leukemia of ages
ranging from 3 to 17 years old who were admitted to Dharmais
Cancer Hospital and Kramat 128 Hospital in Jakarta. Patients
who had been diagnosed with leukemia by a pediatrician, were
entered induction or consolidation phase of chemotherapy, were
able to follow instructions for gargling, and had no habitual use
of probiotic nor mouthwash met the inclusion criteria. Once the
purpose of the study was explained to the parents and guardians,
they all signed a document indicating their informed consent. Patients
were instructed to rinse with a bottle of probiotics for 60
seconds, 2 times a day, within 14 days. One bottle of probiotics
contained 65 ml liquid at a dose of 6.5x109 Lactobacillus casei
CFU/ml.
Oral sample was saliva collected from subjects according to criteria.
Saliva samples were taken at baseline that is before rinsing
with probiotics, then follow-up registrations and samplings were
conducted 7 and 14 days after baseline or rinsing with probiotics.
Minimum 2mL of non-stimulated saliva was collected in a sterile
tube and was immediately cold-transported to the Oral Biology
Laboratory Universitas Indonesia. Saliva sample was centrifuged
for 5 min with 3000RPM then the supernatant was transferred to
another sterile tube and stored at -80°C until used.
Real-time PCR (qPCR) was used for quantification of Candida
albicans. Fungal genomic DNA was obtained using Genezol reagent
concentration and the concentration and quality of the obtained
DNA was determined by by Qubit assay reagents (Invitrogen,
Carlsbad, CA). The genomic DNA samples were dissolved in
Tris-EDTA(TE) buffer and stored at -20°C until processed. Further,
the DNA samples were quantified through a qPCR reaction
Candida albicans specific primers (Table 1). For PCR-quantification,
each sample was run in duplicate on an ABI StepOnePlus
Real-Time PCR System, where a SYBR Green PCR Master Mix
(Applied Biosystems, Foster City, CA, USA) was used according
to the manufacturer’s protocol.
The PCR conditions was set in a final reaction volume of 10 µl,
composed of 50 ng of sample DNA and 1 µM of Candida albicans
specific primers, with thermal cycling condition consisted
of a 10 min initial denaturation at 95°C, followed by 40 cycles
of denaturation at 95°C for 15 s, annealing at 60°C for 60 s, and
elongation at 95°C for 15 s. The qPCR product was visualized as
melting curve (95°C for 15 s, 60°C for 60 s, and 95°C for 15 s),
and cycle threshold value (Ct) were determined automatically by
the qPCR machine.
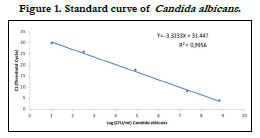
The estimating amount of Candida albicans genomic DNA was
determined by constructing a standard curve with r2 values for
organism tested as shown in Figure 1. To do this, a 5-fold serial
dilution of extracted C. albicans(ATCC 10231) was used. The
fungus number (CFU/ml) was assessed by plating culture dilutions
on Sabouraud agar, and the same strain was used as positive
control while running the qPCR. Therefore, the quantification of
Candida albicans from saliva samples was achieved by plotting the
Ct values against the log of the respective standard curve.
A comparison of the results was done using SPSS version 20.0.
Repeated Measure ANOVA was used to perform statistical analysis
to compare the quantification of Candida albicans at baseline,
after 7 days, and after 14 days mouth rinsing with probiotics. Pvalue
< 0.05 was considered significant.
Results
A standard curve was used to determine the number of Candida
albicans, while melt curve were used to evaluate the amplicon’s
specificity using saliva samples (Figure 2).
In this study, the data showed that Candida albicans was presence
in all saliva samples. The number or quantification of Candida
albicans after converted from its Ct value into CFU (colony
forming unit) using standard curveis shown in Figure 3. In
general, it was observed that in saliva samples of children with
leukemia,decreased number of CFU was found sequentially from
baseline (494.363 + 180.737 CFU/ml), to after 7 days (276.653 +
69.903 CFU/ml), and after 14 days (229.286 + 50.883 CFU/ml) rinsing with probiotics.
Statistically significant differences(p<0.05) were found between
quantities of Candida albicansat baseline and at designated time
intervals during and after rinsing with probiotics containing Lactobacillus
casei. There were significant differences in the quantities
of Candida albicansat every period which are between baseline
and after 7 days of rinsing with probiotics (p=0.005), baseline
and after 14 days of rinsing with probiotics (p=0.002), as well
as between after 7 days and 14 days of rinsing with probiotics
(p=0.016).
Discussion
Numerous studies[11, 16-18] have braced up the idea of using
probiotics in the battle against oral candidiasis. This study was
undertaken to evaluate the relationship between the amount of
Candida albicans, the most common pathogen of oral candidiasis,
and exposure to probiotic bacteria. Previous studies which also
focused on the same issues had mostly been performed in elderly,
thus this study focused in another group of immunosuppressed
patients that are children with leukemia during the induction and
consolidation phase of chemotherapy, as they have increased risk
of fungal infection.[10] Candida albicans is present as commensal
flora in healthy people and becomes the predominant flora in
60% of immunosuppresed people.[19] Chemotherapy, especially
on children, can lead to severe oral candidiasis which has possibility
to spread systematically and become life-threatening, yet there
has not been a sufficient evidence of any antifungal drugs that
may cure fungal infections in the mouth of people with cancer. [20]
In this present study, the major finding was that there was a decrease
of the number of Candida albicans in the subjects’ saliva
following rinsing with probiotics containing Lactobacillus caseiover
the course of 14 days. The results within this study is in
accordance with results from other studies [15-18], which found
that there was significant reduction of Candida cell quantification
after probiotics administration. Previous study proved that the
consumption of probiotic drink containing Lactobacillus casei was
successful in reducing the prevalence of oral Candida and increasing
anti-Candida immunoglobulin A levels in healthy individuals.
[18] Compatible with this study, the use of bacteria with genus
Lactobacillus has been deemed worthy as an alternative method
to return disease-inducing microbiota or opportunistic pathogen
to a healthy, symbiotic, stable commensal equilibrium.[11]
For the mechanism on how probiotics enhance this well-balanced
state, from illness to nutrition, a variety of theories have been
suggested, many unproven as yet. Probiotics may compete against
pathogenic microorganisms for nutrients and receptors on the cell
surfaces, thereby preventing adhesion and colonization of pathogenic
microorganisms, such as Candida albicans, on the mucosal
surfaces.[21] Furthermore, probiotics were found to work against
the main virulence factors of Candida. Probiotics can reduce filamentation
and biofilm development in Candida albicans causing
candida infection to resolve.[11, 22] By suppressing filamentation,
probiotics could assist the host to fight Candida albicans or pathogens
more effectively, as the yeast form of Candida albicansis
more susceptible to phagocytosis.[11]
Apart from the above, it is worth mentioning that the antimicrobial
activity of probiotic bacteria is strain-specific, thus, the
clinical application of probiotics should be aimed at specific pathogens
and their beneficial effects cannot be generalized. Different
microbial probiotic strains could have different effects on the
reduction of Candida albicans.[23] For example, one study stated
that in a mice model, Lactobacillus rhamnosus was more effective
than the Lactobacillus acidophilusin reducing the amount of Candida
spp. colonization levels. [24]
Supported by the obtained results in this study, we can approve
that probiotics have a protective role in Candida infection, notably
colonization. As previously mentioned, it is possible to explain
the anti-Candida properties in various way. However, such positive
effects are highly associated with the administration method,
dosage, the strains of probiotics used, and host factors.
This study has limitations, such as asmall number of sample included
due to difficulty in finding subjects within the inclusive
criteria and short period of evaluation. We recommend that future
studies with larger sample size and longer treatment duration
to be undertaken to evaluate the real effectiveness of probiotics
treatment in Candida infection.
Conclusion
This study demonstrated the effects of rinsing with probiotics on
the number of Candida albicans on saliva of children with leukemia
during the induction and consolidation phase of chemotherapy.
A statistically significant reduction on the amount of salivary Candida albicans after 7 days and 14 days of rinsing with probiotis was
shown. The results of this study suggest that researchers should
conduct further studies to investigate the efficacy and long-term
effects of using probiotics as antifungal prophylaxis and treatment.
Acknowledgement
The researcher would like to thank the patients and parents who
were willing to take part in this research. The researcher also
wants to thank dr. Haridini Intan Setiawati Mahdi, Sp.A(K)Onk,
dr. Edi Setiawan Tehuteru, Sp.A(K),MHA, IBCLC, and Prof.drg.
Endang Winiati Bachtiar, M.Biomed., Ph.Dfor all the help and
guidance that was given. This research was funded by HIBAH
PITTA Universitas Indonesia.
References
-
[1]. Steliarova-Foucher E, Colombet M, Ries LAG, Moreno F, Dolya A, Bray
F, et al. International incidence of childhood cancer, 2001-10: a population-
based registry study. Lancet Oncol. 2017 Jun;18(6):719-731.Pubmed
PMID: 28410997.
[2]. Gupta S, Howard SC, Hunger SP, Antillon FG, Metzger ML, Israels T, et al. Treating Childhood Cancer in Low- and Middle-Income Countries. In: Gelband H, Jha P, Sankaranarayanan R, Horton S, editors. Cancer: Disease Control Priorities, Third Edition (Volume 3). Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2015 Nov 1. Pubmed PMID: 26913338.
[3]. Palumbo MO, Kavan P, Miller WH Jr, Panasci L, Assouline S, Johnson N, et al. Systemic cancer therapy: achievements and challenges that lie ahead. Front Pharmacol. 2013 May 7;4:57. Pubmed PMID: 23675348.
[4]. Alam A, Farooq U, Singh R, Dubey VP, Kumar S, Kumari R, et al. Chemotherapy treatment and strategy schemes: A review. Open Access J Toxicol. 2018;2(5):555600.
[5]. Pearce A, Haas M, Viney R, Pearson SA, Haywood P, Brown C, et al. Incidence and severity of self-reported chemotherapy side effects in routine care: A prospective cohort study. PLoS One. 2017 Oct 10;12(10):e0184360. Pubmed PMID: 29016607.
[6]. Chaveli-López B, Bagán-Sebastián JV. Treatment of oral mucositis due to chemotherapy. J Clin Exp Dent. 2016 Apr 1;8(2):e201-9. PMID: 27034762.
[7]. Al Jaouni SK, Al Muhayawi MS, Hussein A, Elfiki I, Al-Raddadi R, Al Muhayawi SM, et al. Effects of Honey on Oral Mucositis among Pediatric Cancer Patients Undergoing Chemo/Radiotherapy Treatment at King Abdulaziz University Hospital in Jeddah, Kingdom of Saudi Arabia. Evid Based Complement Alternat Med. 2017;2017:5861024. Pubmed PMID: 28270852.
[8]. Berger Velten D, Zandonade E, Monteiro de Barros Miotto MH. Prevalence of oral manifestations in children and adolescents with cancer submitted to chemotherapy. BMC Oral Health. 2016 Oct 3;16(1):107. Pubmed PMID: 27716167.
[9]. Al-Ansari S, Zecha JA, Barasch A, de Lange J, Rozema FR, Raber-Durlacher JE. Oral Mucositis Induced By Anticancer Therapies. Curr Oral Health Rep. 2015;2(4):202-211. Pubmed PMID: 26523246.
[10]. Teoh F, Pavelka N. How Chemotherapy Increases the Risk of Systemic Candidiasis in Cancer Patients: Current Paradigm and Future Directions. Pathogens. 2016 Jan 15;5(1):6. Pubmed PMID: 26784236.
[11]. Matsubara VH, Bandara HM, Mayer MP, Samaranayake LP. Probiotics as Antifungals in Mucosal Candidiasis. Clin Infect Dis. 2016 May 1;62(9):1143-53. Pubmed PMID: 26826375.
[12]. Fijan S. Microorganisms with claimed probiotic properties: an overview of recent literature. Int J Environ Res Public Health. 2014 May 5;11(5):4745- 67. Pubmed PMID: 24859749.
[13]. Hu H, Merenstein DJ, Wang C, Hamilton PR, Blackmon ML, Chen H, et al. Impact of eating probiotic yogurt on colonization by Candida species of the oral and vaginal mucosa in HIV-infected and HIV-uninfected women. Mycopathologia. 2013 Oct;176(3-4):175-81.Pubmed PMID: 23925786.
[14]. Coman MM, Verdenelli MC, Cecchini C, Silvi S, Orpianesi C, Boyko N, et al. In vitro evaluation of antimicrobial activity of Lactobacillus rhamnosus IMC 501(®) , Lactobacillus paracasei IMC 502(®) and SYNBIO(®) against pathogens. J Appl Microbiol. 2014 Aug;117(2):518-27. Pubmed PMID: 24836638.
[15]. Li D, Li Q, Liu C, Lin M, Li X, Xiao X, et al. Efficacy and safety of probiotics in the treatment of Candida-associated stomatitis. Mycoses. 2014 Mar;57(3):141-6. Pubmed PMID: 23952962.
[16]. Ishikawa KH, Mayer MP, Miyazima TY, Matsubara VH, Silva EG, Paula CR, et al. A multispecies probiotic reduces oral Candida colonization in denture wearers. J Prosthodont. 2015 Apr;24(3):194-9. Pubmed PMID: 25143068.
[17]. Kraft-Bodi E, Jørgensen MR, Keller MK, Kragelund C, Twetman S. Effect of Probiotic Bacteria on Oral Candida in Frail Elderly. J Dent Res. 2015 Sep;94(9 Suppl):181S-6S. Pubmed PMID: 26202995.
[18]. Mendonça FH, Santos SS, Faria Ida S, Gonçalves e Silva CR, Jorge AO, Leão MV. Effects of probiotic bacteria on Candida presence and IgA anti- Candida in the oral cavity of elderly. Braz Dent J. 2012;23(5):534-8.Pubmed PMID: 23306230.
[19]. Mishra R, Tandon S, Rathore M, Banerjee M. Antimicrobial Efficacy of Probiotic and Herbal Oral Rinses against Candida albicans in Children: A Randomized Clinical Trial. Int J Clin Pediatr Dent. 2016 Jan-Mar;9(1):25-30. Pubmed PMID: 27274151.
[20]. Worthington HV, Clarkson JE, Khalid T, Meyer S, McCabe M. Interventions for treating oral candidiasis for patients with cancer receiving treatment. Cochrane Database Syst Rev. 2010 Jul 7;2010(7):CD001972. Pubmed PMID: 20614427.
[21]. Ujaoney S, Chandra J, Faddoul F, Chane M, Wang J, Taifour L, et al. In vitro effect of over-the-counter probiotics on the ability of Candida albicans to form biofilm on denture strips. J Dent Hyg. 2014 Jun; 88(3):183-9.Pubmed PMID: 24935148.
[22]. Köhler GA, Assefa S, Reid G. Probiotic interference of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 with the opportunistic fungal pathogen Candida albicans. Infect Dis Obstet Gynecol. 2012;2012:636474. Pubmed PMID: 22811591.
[23]. Mundula T, Ricci F, Barbetta B, Baccini M, Amedei A. Effect of Probiotics on Oral Candidiasis: A Systematic Review and Meta-Analysis. Nutrients. 2019 Oct 14;11(10):2449. Pubmed PMID: 31615039.
[24]. Matsubara VH, Silva EG, Paula CR, Ishikawa KH, Nakamae AE. Treatment with probiotics in experimental oral colonization by Candida albicans in murine model (DBA/2). Oral Dis. 2012 Apr;18(3):260-4. Pubmed PMID: 22059932.
[25]. Bachtiar EW, Bachtiar BM. Relationship between Candida albicans and Streptococcus mutans in early childhood caries, evaluated by quantitative PCR. F1000Res. 2018 Oct 16;7:1645. Pubmed PMID: 30450201.