The Role of CD44 Cancer Stem Cell Marker in the Development and Progression of Lymph Node Metastasis in Oral Squamous Cell Carcinoma
Ahmed M. Hussein1, Asmaa M Zahran2, Mohamed Badawy3, Hany G. Gobran4, Mohamed F. Edrees5, Enas M. Omar6*
1 Lecturer of Oral and Maxillofacial Pathology Department, Faculty of Dentistry, Assiut University, Assiut, Egypt.
2 Associate Professor Clinical Pathology Department, South Egypt Cancer Institute, Assiut University, Egypt.
3 Lecturer of Oral Biology Department, Faculty of Dentistry, Assiut University, Assiut, Egypt.
4 Associate of Oral BiologyDepartment, Faculty of Dentistry, Al-Azhar University (Boys Branch), Egypt.
5 Assistant Lecturer Oral Medicine and Periodontology Department, Faculty of Dentistry, Al-Azhar University, Assiut, Egypt.
6 Lecturer of Oral PathologyDepartment, Faculty of Dentistry, Alexandria University, Alexandria, Egypt.
*Corresponding Author
Enas M. Omar,
Lecturer of Oral PathologyDepartment, Faculty of Dentistry, Alexandria University, Alexandria, Egypt.
Tel: +201225155647
E-mail: inas.magdy@alexu.edu.eg/Inas.magdy@gmail.com
Received: October 05, 2021; Accepted: November 05, 2021; Published: November 09, 2021
Citation: Ahmed M. Hussein, Asmaa M Zahran, Mohamed Badawy, Hany G. Gobran, Mohamed F. Edrees, Enas M. Omar. The Role of CD44 Cancer Stem Cell Marker in the
Development and Progression of Lymph Node Metastasis in Oral Squamous Cell Carcinoma. Int J Dentistry Oral Sci. 2021;8(10):4917-4922. doi: dx.doi.org/10.19070/2377-8075-21000994
Copyright: Enas M. Omar©2021. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: Oral squamous cell carcinoma (OSCC)management is challengingdue to high tendency of local invasion and
metastasis.Cancer stem cells hold highsignificanceas theyhaveself-renewal ability, which further allowscancer progression and
metastasis.Hence, it is crucial to evaluate different specialised markers for stem cells,such as CD44,to detect their role in tumour
metastasis.Flow cytometry (FCM) offers a quick and automated assessment of ploidy status andcell proliferation of the
neoplasm by resolvingthe nuclear DNA contents.
Aim of the study: The objective of thispaperis to evaluate theCD44 expression and analyseDNA content by FCM to predict
the expansion of lymph node metastasis in patients with OSCC.
Material and Methods: 50paraffin-embedded tissues of metastatic and non-metastatic lymph node OSCCwereimmunostainedby
CD44for assessing cancer stem cell activity in each lesion. Furthermore, each selected tissue underwent flow
cytometric analysis to demonstratethe DNA activity between the tested groups.
Results: The CD44 expression in OSCCsshowed a marked difference between the metastatic and non-metastatic lymph node
cases. Furthermore, flow cytometric analysis of the DNA parameters between the tested groups revealed a powerful difference
of DNA ploidy. The S-phase fraction(SPF) between the groups showed no compelling result.All specimens had a higher
CD44 expression, aneuploid DNA content and high SPF, whichdemonstrateddepositsof cervical metastatic lymph nodes.
Conclusions: The CD44 and the flow cytometric analysis of the DNA ploidy correlationoffer a significantprediction to
determine the OSCCcompetence .
2.Introduction
3.Materials and Methods
3.Results
4.Discussion
5.Conclusion
5.References
Keywords
CD44; Cancer Stem Cell; Flow Cytometry; DNA Ploidy; S-Phase Fraction..
Introduction
Oral squamous cell carcinoma (OSCC) is, particularly, the mostfamiliaroral
head and neck cancer worldwide. It always demonstratea
poor prognosis becauseof its late-stage diagnosis, local
invasion and the recurrence of primary carcinomas. A study has
revealed that the presence of lymph node metastasis is considered
as the eventual and pivotal prognostic signal of survival and recurrence.[
1] The assessment of lymph node status plays a crucial
role in the treatment plan and the prediction of the patient survival.
However,a part ofhidden lymphatic metastases is still missed
in investigation, which contribute in reducing the survival rate.[2,
3] However, in early-stage OSCC, the adoption of elective neck
dissection has been questionable during the previous several decades.
The regional lymph node metastasis through the pathologic
evaluation is recognised in only a few patients. For those patients
without lymph metastasis, the undesirable cosmetic and functional
effects along with an increasing morbidity of neck dissection
should be avoided. Thus, it is essential to meticulously predict
lymph node metastasis before the surgery [4]. This phenomenon
indicates the need for establishing other methods to determine
the propensity for metastasis.
One of the theses regarding oral carcinogenesis and metastasis
state that the neoplasm growth depends on cancer stem cells with
self-renewal abilities that can advocate cancer initiation, advancementand
metastasis.[5] Explicit markers for these cells such as
CD44 were investigated to promote a profound understanding
for CSCs’actions in carcinogenesis and metastasis.[6] The CD44
is a cell surface glycoprotein assuming as a dominant receptor
for hyaluronic acid. It participates in physiologic and pathologic
processes such as lymphocyte homing, wound healing, angiogenesis
and malignant diseases. It is also associated in cell attachment
and migration.[6] The CD44 antigen is marked by the CD44 gene
loaded on chromosome 11. Additionally, the CD44 is believed to
be involved in cancer progression and metastasis as a regulator of
growth, survival, differentiation, and migration.[7, 8]
A great deal of interest has been directed towards using flow cytometric
DNA analysis as an objective tool to study the natural
history of SCC of the head and neck. Tumour DNA content is
asserted to be one of the prognostic and metastatic indicators in
this cancer.Several investigators have studied the same with respect
to lymph node metastasis.[9-11] DNA ploidy has proven
to be a useful prognostic indicator in various neoplasms.[12] The
analysis of solid lesions by flow cytometry (FCM) permits rapid,
objective, quantitative evolution and proliferative activity of cellular
DNA content.[13] Neoplasms are usually classified according
to their ploidy status into diploid typeswith a normal amount
of DNA (2N) and aneuploid ones with an abnormal amount of
DNA. Besides, the FCM also provides some assessment of cellular
proliferative activity, defined by S-phase fraction (SPF), all
of which may add a new dimension to the present pathologic and
metastatic potentials of malignancy [14]. Computer-assisted cell
cycle analysis by FCM provides the sensitivity for exposure near
diploid/aneuploid peaks. FCM also possessesthe advantage of allowing
retrospective studies of paraffin-embedded tissue samples
as well as from fresh or frozen tissue samples.[15] However, there
are few reports on the relationship of flow cytometric analysis
of nuclear DNA content of oral carcinomas with regional lymph
node metastasis.
The objective of this research is to study the CD44 expression
of CSCs and conduct flow cytometric analysis of nuclear DNA
content of OSCC. This research further evaluates the diagnostic
significance of these methods in anticipating the possibility of
cervical lymph node metastasis.
Materials and Methods
This research was reviewed and approved by the institute’s ethical;
board (IORG#:IORG0008839). The cases were retrospectively
retrieved between 2015-2020 and obtained from incisional or excisional
primary tumor biopsies during the same period of time
from files of Oral Pathology Department, Faculty of Dentistry,
Alexandria University over the last five years. Clinical data, including
age, sex, site were obtained from the original pathology
reports. Patients with OSCC who had at least one pathologically
metastatic node were evaluated in a ratio of 50:50 (Group I). The
remaining lesions with no data of any regionally mph node metastasis
were categorised into Group II. Clinical staging, pathological
differentiation and mode of infiltration of the primary carcinomas
were defined based on the Union for International Cancer
Control TNM Classification of Malignant Neoplasms and the
World Health Organization’s classification.[16, 17]
In this study’s tissue samples, one section having a thickness of
5 µmwas cut from each block and stained with haematoxylin and
eosin for the verification of diagnosis. Importantly, histopathological
grading reassures that the neoplasm tissue constitutes
more than 70% of the section, with minimal haemorrhagic andnecrotic
foci. This step is essentialin obtaining accurate results
and avoiding errors produced by analysing normal, inflammatory
or necrotic tissues.
The tissue paraffin blocks were stained by an anti–CD44 antibody
immunohistochemistry marker (Abcam, Cambridge, UK)
to compare its different expressions among metastatic and nonmetastatic
lymph node OSCC. The staining steps were conducted
while adhering to the universal immunostaining protocols. The
strength of the CD44 immunoreaction was evaluated in terms of
both means area (%)and optical density by using the image analyser
(Faculty of Oral and Dental Medicine, South Valley University).
Each specimen was marked according to the power of the
nuclear and cytoplasmic staining: no staining, 0; weak staining, +;
moderatestaining, ++; and strong staining, +++.
From each block, 3 pieces having a thickness of 50 µmwere cut
and transmitted to the FCM Unit, Clinical Pathology Department,
South Egypt Cancer Institute, Assiut University for flow cytometric
analysis using Becton–Dickinson (B–D) FACS Calibur flow
cytometer (USA). Specimens were stained by thecycle test TMplus
DNA Reagent Kit (BD Biosciences. For each selected block,
thickness of 50 µm were placed into a labelled glass culture tube
with dimensions of 16 × 125 mm. Nuclear suspensions of solid
lesions were prepared using a modified version method18 The
samples with a single G0/G1 peak were classified as DNA diploid.
If two discrete G0/G1 peaks were present with an abnormal
G0/G1 peak containing a minimum of 15% of the total events
and having a corresponding G2/M peak, then the neoplasms
were considered as DNA aneuploidy.[19] The DNA index (DI)
was recorded by the calculation programme for the DNA analysis
system. The SPF is the fraction of the full cell residents that are
present in the S-phase of the cell cycle and is usually asserted as
a ratio. The cut-off for the SPF was set as the mean ±2 standard
deviation (SD) and considered as either being low or high.
The data were collected, tabulated and statistically analysed using
the SPSS system (release 11.0 software). All results were expressed
as mean ± SD. One-way ANOVA was employed to test
the data between the examined neoplasms. It was also used to
analyse the mean CD44 area (%) and the optical density of immunohistochemical
results. The FCM variables between the research
groups were compared using the Mann–Whitney U test
and Kruskal–Wallis test. Chi-squared (±2) test was performed tocomparethe
categorical data. P< 0.05 was considered significant
in all the statistical results..
Results
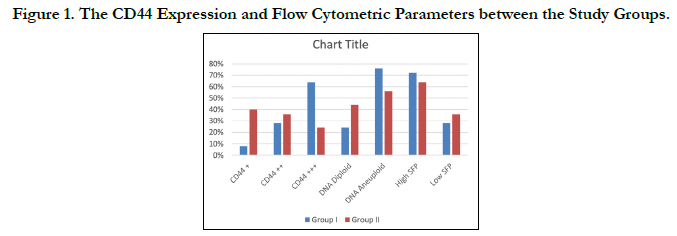
Thisstudy was performed on 50 OSCC specimens at different
clinical stages with variable histological grades. Half of the specimens
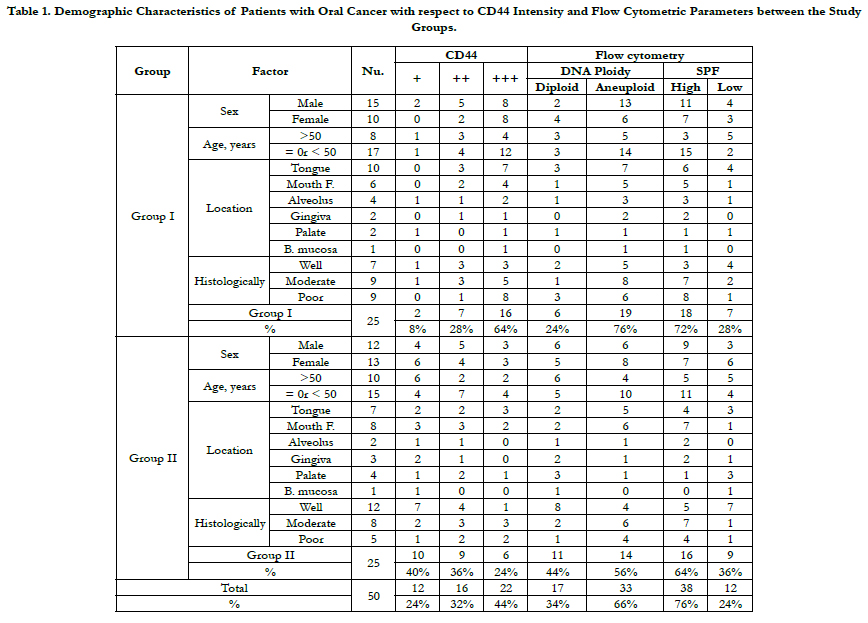
with positive lymph node metastasis were identified (Figure
1). The increase in CD44 expression and aneuploidy state was powerfully associated with a higher stage and grade of disease,
where as no relationship observed between CD44 immunoreactivity
or aneuploidy state and patients’ gender, age and neoplasm
location (Table 1).
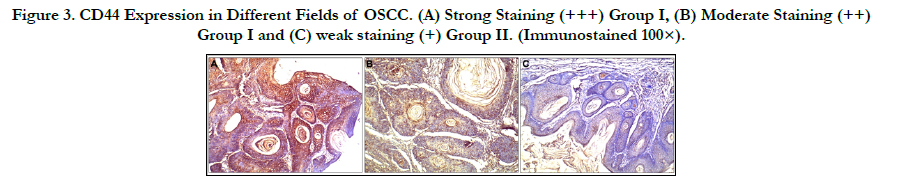
The immunoreaction to CD44 in the different tested groups
showed variations in both mean area (%) and the optical density
(Figure 2).In Group I, the mean CD44 area was 66.14 ± 7.54%,
and the mean CD44 optical density was 74.46 ± 11.58. A total
of16 specimens (64%) exhibited strong staining (+++); 7 tumours
(28%) exhibited moderate staining (++) and only 2 lesions
(8%) exhibited weak staining (+). In Group II, the mean CD44
area was 44.06 ± 7.43%, and the mean CD44 optical density was
54.35 ± 9.52. A total of 10 neoplasms (40%) showed weak staining
(+);9 tumours (36%) showed moderate staining (++) and 6
lesions (24%) expressed strong staining (+++).The differences in
both mean CD44 area (%)and optical density were highly statistically
significant (p <0.0001), as shownin the comparison between
Group I and Group II. As expected, the expression of CD44
was lower in non-metastatic cases (Group II) as compared to the
metastatic lesions (Group I). Despite this result, it was noted that
a decreased level of CD44 should not be considered exclusively
for the possibility of lymph node metastasis.
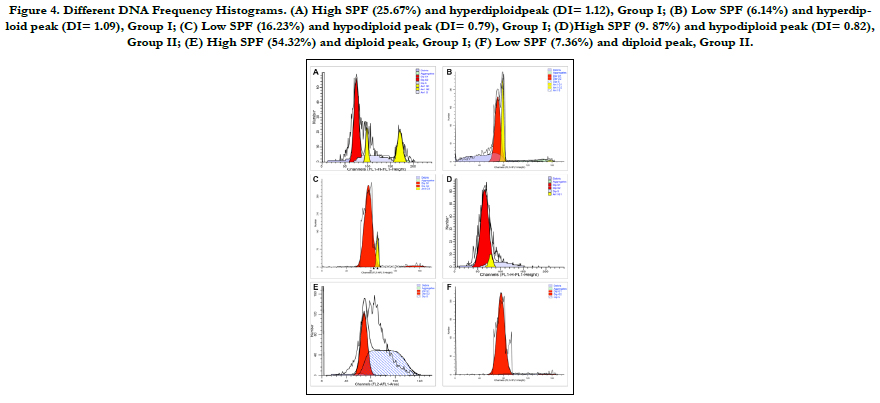
In the flow cytometric analysis (Figure 3), 33 (66%) lesions were
found to have aneuploid cell populations and the remaining 17
tumours had a diploid cell population. Aneuploidy was observed
in 19 out of 25 (76%) specimens in Group I and in 14 out of 25
(56%) tumours in Group II. The aneuploid neoplasms further
were divided into: hyperdiploid with DI ranging from 1.05 to 1.82
with a mean of 1.45 (13 in Group I and 7 in Group II) and hypodiploid
with DI ranging from 0.71 to 0.97 with a mean of 0.85
(6 in Group I and7 in Group II). The difference in diploid and
aneuploid DNA patterns (the ploidy state) between Group I and
Group II was statistically significant (p= 0.002). There is no symbolic
difference in the numbers of hyperdiploid and hypodiploidcases
between Group I and Group II (p= 0.657). The SPF values
calculated for Group I ranged between 6.14% and 67.21% with a
mean of 24.77%,whereas the SPF values calculated for Group II
ranged between 4.49% and 43.19% with a mean of 15.35%. The
S-phase values were furthered classified into high and low. About
72% (18 out of 25) of specimens in Group I had high SPF value
(numbers of cells in SPF were equal or more than 24.77%), and
28% (7 out of 25) of tumours had low SPF value. In Group II lesions,
nearly 64% (16/25) of cases had high SPF (number of cells
in SPF were more than 15.35%), and 9 lesions(36%) had low SPF
values. There is noimportant difference in the mean SPF value of
Group I and Group II specimens (p= 0.537).
There is a high significance difference (p <0.0001) between the
specimens with strong CD44 expression, aneuploid content and
high SPF (64%, in 16 out of 25) in Group I and the same examined
lesions (24%, in 6 out of 25) in Group II. This research result
determined that the CD44 expression collaborates with the FCM
analysis results of the tumour DNA content. Furthermore, this
result establishes that CD44 expression is astrong diagnostic indicator
for anticipating the qualification of oral cancer to produce
cervical lymph node metastasis.
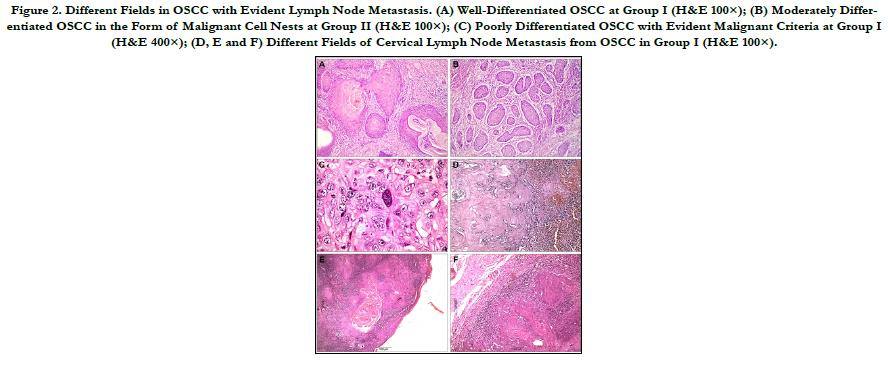
Figure 2. Different Fields in OSCC with Evident Lymph Node Metastasis. (A) Well-Differentiated OSCC at Group I (H&E 100×); (B) Moderately Differentiated OSCC in the Form of Malignant Cell Nests at Group II (H&E 100×); (C) Poorly Differentiated OSCC with Evident Malignant Criteria at Group I (H&E 400×); (D, E and F) Different Fields of Cervical Lymph Node Metastasis from OSCC in Group I (H&E 100×).
Figure 3. CD44 Expression in Different Fields of OSCC. (A) Strong Staining (+++) Group I, (B) Moderate Staining (++) Group I and (C) weak staining (+) Group II. (Immunostained 100×).
Figure 4. Different DNA Frequency Histograms. (A) High SPF (25.67%) and hyperdiploidpeak (DI= 1.12), Group I; (B) Low SPF (6.14%) and hyperdiploid peak (DI= 1.09), Group I; (C) Low SPF (16.23%) and hypodiploid peak (DI= 0.79), Group I; (D)High SPF (9. 87%) and hypodiploid peak (DI= 0.82), Group II; (E) High SPF (54.32%) and diploid peak, Group I; (F) Low SPF (7.36%) and diploid peak, Group II.
Table 1. Demographic Characteristics of Patients with Oral Cancer with respect to CD44 Intensity and Flow Cytometric Parameters between the Study Groups.
Discussion
Oral cancerexhibits an aggressive behaviour along with a high incidence of nodal metastasis, even in the initial stages, which always
causes a poor prognosis. [20] The CSCs hypothesis states
thatCSCs exerted both regional and systemic effects on the cancer
growth and metastasis.[21] CD44 was proposed as the ideal
CSCs marker. The CSCs population identified by CD44 antibody
expression was linked and parallel with the carcinogenesis process
activity. It further contributed to aggressive cancer phenotypes.
[22] This study’s findings demonstrated a link between higher
expression levels of CD44 and cervical lymph node metastasis
of OSCC. Furthermore, this link has been shown to predict a
poor survival and prognosis in patients with cancer. The CD44
expression plays a performative role in cancer aggressiveness and
metastasis. A marked increase in the CD44 area (%) and optical
density was recorded in the examined tissues with lymph node
metastatic deposits. The marker expression demonstrated a highly
statistically value in the comparison between metastatic and nonmetastatic
lymph node OSCC. A high expression of CD44 could
provide relevant information for the high competence of malignant
cellsthat promote the progress of metastatic deposits.
In agreement with the our research results, Mirhashemi et al. observed
a higher expression of CD44 and CD24 in OSCC, and
revealed the possibility of malignant transformation.[23] Additionally,
Judd et al. noted that the weak CD44 expression caused a
delay in the carcinoma growth and metastasis.[24] Moreover, Cohen
et al.tested the CD44 expression thatpresented as a prognostic
aspect in oropharyngeal carcinomas.[25] Furthermore, comparable
results reported by de Andrade et al. identified that the
tissue cells with a strong CD44 expression had a higher capacity
to form malignance.[26] Likewise, a study oforal cancer cell lines
conducted by Ghuwalewala et al. revealed thatthe cell population
with an intense CD44 expression enhanced a more tumorigenic
potential along with invasive and metastatic skills.[27] Additionally,
Paulis et al.concluded that the CD44 expression increased the
aggressiveness of cancer cells behaviour.[28] Li et al.suggested
that intense CD44 expression correlates to the advanced tumour
grades, recurrence and poor prognosis.[29] This observation goes
in line with the results of our research. In contrast, Krump et
al. demonstrated that there are no serious differences in CD44
reactions between the different carcinoma grades in the oral cavity.
[30] This shortening in the appearance of CD44 may be attributed
to the improper selection of the examined tissues that
may have massive areas of inflammation, further leading to a false
result in the CD44 expression. Moreover, the difference in the
examined tissue and the sorting techniques with that of immunohistochemistry
for identifying the CD44 expression in tissue such
as Western blotting and flow cytometric assessments may have
validated different sorting results.
This study demonstrated that the differences in DNA ploidy was
highly statistically significant (p <0.0001) as revealed in thecomparison
between Group I (metastatic lymph node OSCC) and
Group II (non-metastatic lymph node OSCC). The research results
are compatible with El-Deftar et al.’s findings.[31] Furthermore,
Hayry et al.’s resultsindicate that the nuclear morphometric
features and analysis of DNA ploidy of the nodal tissue by FCM
mainly help as the prognostic metastatic markers of oral cancer.
[32] Supporting data published by Kamphues et al. reported that
the DI represents an independent prognostic marker both postand
preoperatively. It might become a potential tool in the preoperative
decision-making process.[33] Furthermore, Jagric et al.
concluded that FCM is a rapid, cost-effective, widely obtainable
and highly distinct method for sentinel lymph node metastases.
Therefore, it cannot be recommended as the onlytest for detecting
lymph node metastases beforesurgeries.[34] Moreover,Acosta
et al.concluded that the finding of positive aneuploid cells using
FCM strongly indicates the presence of carcinoma cells.[35]. Additionally,
Missaoui et al. showed that DI and SPF appear helpful
in making the distinction between benign and malignant lesions,
and aneuploidy appears to be more interesting in the prognosis
evaluation of these neoplasms.[36] However, Ludovini et al. do
not support the prognostic aspect of DNA ploidy to spot patients
with more aggressive tumours who are at a high risk for disease
relapse and metastases.[37] Furthermore, Zargoun et al. reported
that DNA ploidy alone was not specific and may not be a good
tool to evaluate prognosis or metastatic progression in oral cavity
carcinomas.[38] This result is in agreement with our findings,
which recorded that the collaboration between the CD44 immunohistochemical
expression and the DNA analysis by FCM was
more effective in predicting the capability of the malignant cell to
promote lymph node metastases.
Our finding showed that there was no important relationship
between the SPF of the metastatic and non-metastatic lymph
node cases. This result agrees with Zahran et al.’s results, which
expressed that theDNA aneuploidy may be a key indicator for
tumour activity and malignancy in salivary gland tumours with
no significant SPF value in evaluating tissue activity.[39] Similarly,
Pinto et al. report a borderline significance of SPF with respect
to the overall survival and loco-regional lymph node metastasis
of papillary thyroid carcinoma.[40] In different circumstances,
Pervez et al.found that the SPF was a more reliable marker in
anticipating the axillary lymph node metastases in breast carcinomas.[
41] Oya et al. also demonstrated that theDNA ploidy is
heterogeneous within a cancer, whereas SPF is relatively stable
and can be correlated with regional metastasis in oral cancer.[42]
Lymph node metastasis is a convoluted progression of events.
Initially, cancer cells penetratetheir adjacent tissues and move
throughthe lymphatic vessels. Finally, they are carried to the cervical
lymph nodes where they must be deposited and grow to form
metastatic lesions. The chance of evolution of each cell population
would be higher with heterogeneous tumours.[43, 44] At the
time of diagnosis, malignant neoplasms have undergone various
changes during progression and usually contain subpopulations
of cells with different biologic features. Obviously, aneuploid carcinomais
more heterogeneous than the diploid one in terms of
cell populations. This may be the reason for the higher incidence
of lymph node metastasis shown by the aneuploid carcinomasas
compared to the diploid one.
Conclusions
In conclusion, the immunoexpression of CD44 was found in
all the OSCC samples. The high CD44 expression was compellingand
associated with a higher stage of lymph node metastasis.
The state of DNA ploidy can possibly advance our prediction
oforal cancer strength to establishlymph node metastasis.
Further studies regarding the anticipation of the oral cancer metastasiswill
definitely increase therapeutic success while effectively
decreasing morbidity and mortality of OSCC.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
References
-
[1]. Canay S, Kocadereli I, Akca E. The effect of enamel air abrasion on the retention
of bonded metallic orthodontic brackets. Am J Orthod Dentofacial
Orthop. 2000; 117: 15¬19. Pubmed PMID:10629515.
[2]. Buonocore MG. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J DentRes 1955; 34: 849¬853. Pubmed PMID:13271655.
[3]. Vasei F. Effect of chitosan treatment on shear bond strength of composite to deep dentin using self-etch and total-etch adhesive systems. Brazilian Dental Science. 2021;24(2).
[4]. Van Meerbeek B, Inouse S, Perdiago J, Lambrechts P, Vanherle G. Enamel and dentin adhesion. Fundamentals of Operative Dentistry. A Contemporary Approach. Chicago: Quintessence, 178-235, 2001.
[5]. Saroglu I, Aras S, Oztas D. Effect of deproteinization on composite bond strength in hypocalcified amelogenesis imperfecta. Oral Diseases 2006;12 (3): 305-308.
[6]. Aras S¸, Ku¨c¸u¨kes¸men C¸, Ku¨c¸u¨kes¸men HC. Influences of dental fluorosis and deproteinization treatment on shear bond strengths of composite restorations in permanent molar teeth. Fluoride2007; 40(4): 290-291.
[7]. Atkins CO Jr, Rubenstein L, Avent M. Preliminary clinical evaluation of dentinal and enamel bonding in primary anterior teeth. J Pedod 1986; 10: 239¬246.
[8]. Espinosa R, Valencia R, Uribe M, Ceja I, Saadia M. Enamel deproteinization and itseffect on acid etching: An in vitro study. J Clin Pediatr Dent 2008;33:13-9.
[9]. Venezie RD, Vadiakas G, Christensen JR, Wright JT. Enamel pretreatment with sodium hypochlorite to enhance bonding in hypocalcified amelogenesis imperfecta: Case report and SEM analysis. Pediatr Dent 1994;16: 433-436.
[10]. Ahuja B, Yeluri R, Baliga S, Munshi AK. Enamel deproteinization before acid etching – A scanning electron microscopic observation. J Clin Pediatr Dent 2010;35:169-72.
[11]. Espinosa R, Valencia R, Uribe M, Ceja I, Cruz J, Saadia M, et al. Resin replica in enamel deproteinization and its effect on acid etching. J Clin Pediatr Dent 2010;35:47-51.
[12]. Go´mez S, Bravo P, Morales R, Romero A, Oyarzu´n A. Resin penetration in artificial enamel carious lesions after using sodium hypochlorite as a deproteinization agent. JClin Pediatr Dent 2014;39:51-6.
[13]. Aras S, Ku¨c¸u¨kes¸men C, Ku¨c¸u¨kes¸men HC, So¨nmez IS. Deproteinization treatment on bond strengths of primary, mature and immature permanent tooth enamel. J Clin Pediatr Dent. 2013;37:275-9.
[14]. Abdelmegid FY. Effect of deproteinization before and after acid etching on the surface roughness of immature permanent enamel. Niger J Clin Pract. 2018 May;21(5):591-596. Pubmed PMID: 29735859.
[15]. Hasija P, Sachdev V, Mathur S, Rath R. Deproteinizing Agents as an Effective Enamel Bond Enhancer-An in Vitro Study. J Clin Pediatr Dent. 2017;41(4):280-283. PubmedPMID: 28650791.
[16]. López-Luján NA, Munayco-Pantoja ER, Torres-Ramos G, Blanco-Victorio DJ, Siccha-Macassi A, López-Ramos RP. Deproteinization of prim1/ary enamel with sodium hypochlorite before phosphoric acid etching. Desproteinización del esmalte primario con hipoclorito de sodio antes del grabado con ácido fosfórico. Acta Odontol Latinoam. 2019;32(1):29-35.
[17]. Bahrololoomi Z, Kabudan M, Gholami L. Effect of Er:YAG Laser on Shear Bond Strength of Composite to Enamel and Dentin of Primary Teeth. J Dent (Tehran).2015;12(3):163-170. Pubmed PMID:26622267.
[18]. Heyman HO, Ritter AV, Roberson TM. Introduction to composite restorations. In: Roberson TM, Heyman HO, SwiftE. Sturdevant's Art and Science of operative dentistry. St.Louis: Mosby Elsevier, 6: 216-228, 2013.Van Meerbeek B, De Munck J, Yoshida Y, Inoue S, Vargas M, et al. Buonocore memorial lecture. Adhesion to enamel and dentin: current status and future challenges. Oper Dent 2003;28: 215-235. Pubmed PMID:12760693.
[19]. Kodaka T, Kuroiwa M, Higashi S. Structural and distribution patterns of surface Prismless enamel in human permanent teeth. Caries Res 1991;25 (1): 7-20. Pubmed PMID: 2070383.
[20]. Ramakrishna Y, Bhoomika A, Harleen N, Munshi AK. Enamel deproteinization after acid etching-Is it worth the effort?. Dentistry. 2014;4(2):1-6.
[21]. Sirisha K, Rambabu T, Shankar YR, Ravikumar P. Validity of bond strength tests: A critical review: Part I. J Conserv Dent. 2014;17(4):305-311.
[22]. Van Meerbeek B, Peumans M, Poitevin A, Mine A, Van Ende A, Neves A, et al. Relationship between bond-strength tests and clinical outcomes. Dent Mater. 2010;26:e100–21. Pubmed PMID: 20006379.
[23]. Harleen N, Ramakrishna Y, Munshi AK. Enamel deproteinization before acid etching and its effect on the shear bond strength – An in vitro study. J Clin Pediatr Dent. 2011;36:19-23. Pubmed PMID:22900439.