Role of Salivary Biomarkers in Caries Risk Assessment
Omair M. Bukhari1, Amin A. Marghalani2*
1 Department of Preventive Dentistry, Faculty of Dentistry, Umm Al-Qura University Saudi Arabia.
2 Department of Oral & Maxillofacial Surgery, Faculty of Dentistry, Umm Al-Qura University Saudi Arabia.
*Corresponding Author
Amin A. Marghalani,
Department of Oral & Maxillofacial Surgery, Faculty of Dentistry, Umm Al-Qura University Saudi Arabia.
Tel: +966535252200
E-mail: aaamarghalani@uqu.edu.sa
Received: September 18, 2021; Accepted: November 13, 2021; Published: November 23, 2021
Citation: Omair M. Bukhari, Amin A. Marghalani. Role of Salivary Biomarkers in Caries Risk Assessment. Int J Dentistry Oral Sci. 2021;8(11):5104-5108. doi: dx.doi.org/10.19070/2377-8075-210001027
Copyright: Amin A. Marghalani©2021. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: This study was performed to quantify the association of salivary alpha-defensins HNP1 to -3 with caries activity
in children and identify salivary defenses that may reduce or stop dental caries development.
Methods: At patients' convenience, unstimulated whole saliva was collected from patients visiting the dental teaching hospital.
Laboratory investigations were performed to evaluate salivary PH, a-defensin, total antioxidant, total protein, and cariogenic
microorganisms’ levels. After that, participants' dental records were reviewed for dental caries activity.
Results: The results showed a significant association between caries activity and different salivary biomarkers (salivary pH,
a-defensin, streptococcus mutans, and lactobacilli). For every one-unit increase in salivary pH, the odds of having active dental
caries decreased by 89% (OR=0.11, P=0.018), and for every one-unit increase in a-defensin the odds of having active dental
caries decreased by 25% (OR=0.75, P=0.004), and the odds of having active dental caries for the participant with salivary
streptococcus mutans or lactobacilli levels more than or equal to 105 cfu/ml were 8.8 (OR=8.8, P=0.001) and 22 (OR=22,
p=0.001) times higher than those with levels less than 105 cfu/ml respectively. On the other hand, there were no significant associations
between salivary total protein, total antioxidant, and caries activity. However, for every one-unit increase in salivary
total protein, the odds of having active dental caries increased by 211% (OR=2.11, P=0.072).
Conclusion: The levels of a-defensin, salivary pH, and cariogenic bacteria can be used as risk assessment markers for screening
and assessing caries susceptibility.
2.Introduction
3.Materials and Methods
3.Results
4.Discussion
5.Conclusion
5.References
Keywords
Risk Assessment; Dental Caries; Prevention; Preventive Dentistry; Salivary Biomarkers.
Introduction
Dental caries is one of the most common chronic childhood
problems worldwide. Evidence indicates that it is a result of bacterial
infection,[1]; however, it is also modified by several factors,
including the host (saliva and teeth) and oral microflora (plaque
and bacteria).[2, 3] Salivary defense systems (physiochemical characteristics)
are crucial in preventing dental caries such as buffering
capacity, pH, varying protein concentrations, salivary flow rate,
and secretion of antimicrobial peptides (AMPs).[4]
The most outstanding AMPs are the defensins. Depending on the
form of cysteine combination, two types of defensins are documented,
namely the a and b defensins.[5] The defensins HNP1 to
-3 have been expressed in gingival crevicular fluid, are identified in
neutrophils, and have a role in nonoxidative bacterial death. This
form of secretion suggests that the defensins may prevent dental
caries and protect oral mucosa.[6, 7] Additionally, the defensins
have broad antimicrobial action against gram-positive and gramnegative
bacteria.[8] Therefore, the expression of a-defensins in
saliva may create an extra layer of protection by providing a natural
antibiotic barrier.
The normal concentration for salivary HNP1-3 in a healthy individual
ranges from being undetectable to ~12µg/ml, HNP1–3
elevated with oral inflammation and loose or exfoliating teeth.[9,
10] However, there is no consensus about the relationship between
salivary HNP1-3 levels and dental caries. Some researchers
reported that salivary HNP1-3 levels had a protective effect
against dental caries.[11-14] On the other hand, Toomarian et
al. reported no association between salivary HNP1-3 and dental
caries.[15] Also, in 2109, Devarajan and Somasundaram, via a systematic review, reported insufficient evidence regarding the
relationship between HNP1-3 and dental caries.[5]
The present study aims to quantify the association of salivary a
defensins HNP1 to -3 with caries activity in children and identify
salivary defenses that may reduce or stop dental caries development.
Materials And Methods
Study Design
The present study was a cross-sectional study where participants
had a clinical dental examination to determine caries activity and
then provided a saliva sample to measure different salivary components.
Setting
The present study was carried out at the dental teaching hospital
of Umm Al-Qura University in Makkah city, Saudi Arabia.
Subjects visiting the dental teaching hospital participated at their
convenience. However, individuals with medical conditions or individuals
who were utilizing any drugs or mouthwashes during the
most recent two months before participation in the study were
excluded.
Data Sources and Measurements
Dental Clinical examination: During the registration process,
each patient was given a complete dental exam by a qualified
dentist using a dental mirror, explorer, and orthopantomogram
(OPG) radiographs at the dental teaching hospital. Bitewing radiographs
were taken whenever needed to overcome any uncertainties.
Examination of all teeth and surfaces for dental decay
was done. The investigator analyzed and reviewed 250 patients'
dental health records to get the caries activity.
Saliva samples: Participants were told not to eat for one hour
before the examination. Two ml of unstimulated saliva were collected
from every participant by asking him\her to allow passive
saliva flow into a clean container. The collected sample was used
to determine the total protein, total antioxidant, antimicrobial
peptide (a-defensin 1-3), PH, S. Mutans, and lactobacilli levels.
Salivary pH: The salivary pH values were digitally recorded using
a pH meter (HORIBA Ltd, Japan), following the manufacturer's
instructions.
Salivary S. mutans and lactobacilli levels assessment: The
concentration of S. mutans and lactobacilli was determined using
Caries Risk Test kits (CRT bacteria, Vivadent, Schaan Liechtenstein,
Germany) following the manufacturer's instructions.
Storage of saliva samples: 5 µl of the Nonidet P40 was added
to each saliva sample to obtain the final concentration of 0.1%.
Vials which contain the saliva samples were tightly sealed and
stored at – 80 Co for later analysis.[16]
Protein and antioxidant assessment: Levels of salivary total
protein were examined using an auto-analyzer (Technicon RAXT,
USA) as indicated by the Biuret strategy.[17] Levels of total antioxidant
were dictated by the response of antioxidants in the saliva
sample with a characterized measure of hydrogen peroxide
as indicated by producer's guidelines (Biodiagnostic, Dokki, Giza,
Egypt).
Antimicrobial peptide (a-defensin) assessment: Following
manufacture's guidelines (Hycult Biotechnology, Uden, The
Netherland), the concentration of HNP (1-3) was evaluated using
ELISA kits.
Bias
Non-response could be an issue influencing the findings of a
cross-sectional study with a convenience sample and lead to a bias
of the measures of the outcome. This may be a critical issue or
bias when the participants differ from those who didn't participate.
Sample Size Calculation
Logistic regression of a binary response variable (caries-free) on a
continuous variable (a-defensin) with a sample size of 167 observations
achieves 90% power at a 0.05 significance level to detect a
change in probability of having dental caries by 10% at the mean
of a-defensin when a-defensin is increased to one standard deviation
above the mean. This change corresponds to an odds ratio of
3.353. An adjustment was made since a multiple regression of the
independent variable of interest on the other independent variables
in the logistic regression obtained an R-Squared of 0.100.[18]
Statistical Analysis
Descriptive characteristics are reported as means, standard deviations
for continuous variables, and numbers and percentages for
categorical variables. Categorical variables were tested using chisquare
statistics for bivariate analyses, while continuous variables
were tested using a two-sample t-test with equal variances. For
multivariable analysis, a model was developed using multivariable
logistic regression to predict the odds of having active dental caries
and inspect the concurrent association of salivary biomarkers
on caries activity. The model was adjusted for age and gender. To
determine what variables to include, since the goal was exploratory
in nature, a backward stepwise logistic regression was used
with a probability of removal of 0.2 and a probability of entry of
0.15. All statistics were performed in STATA software (Version
14.2; Stata, college station, TX.). All p-values were two-tailed and
interpreted at 0.05 significance level.
Ethical Considerations
After obtaining the ethical approval from the Research Ethics
Committee, written informed consents were obtained from the
participants after understanding the aim of the study. The work
described in this article has been carried out in accordance with
The Code of Ethics of the World Medical Association (Declaration
of Helsinki).
Results
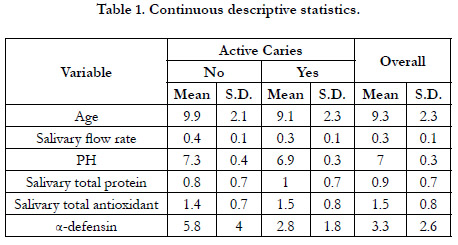
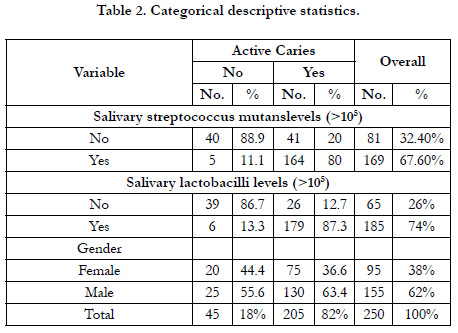
Of the 250 participants, 45 were caries-free (18%). The overall mean age was 9.3 years (SD +/- 2.3). The mean age for caries-free
children was 9.9 years (SD +/- 2.1), while it was 9.1 years (SD
+/- 2.3) for children with active caries. Males composed 62% of
the participants. Descriptive statistics are summarized in Table 1
for continuous variables and summarized in Table 2 for categorical
variables.
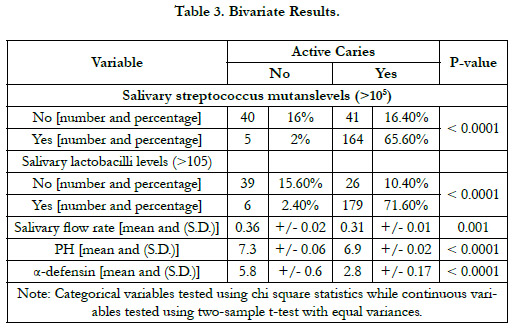
On the bivariate level, salivary flow rate, salivary PH, Salivary
a-defensin, salivary S. mutans levels, and salivary lactobacilli levels
differed significantly among caries-free children when compared
to children with dental caries (Table 3).
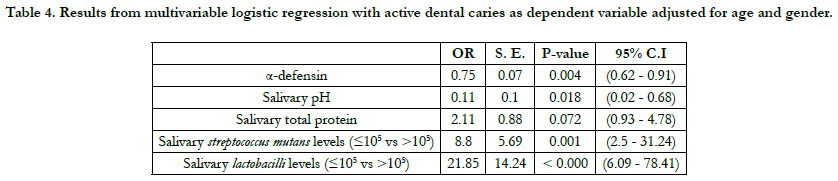
The multivariable findings are summarized in Table 4. The fitted
model shows that, while adjusting for age and gender; and
holding salivary PH, salivary total protein, salivary S. mutans, and
lactobacilli levels at fixed values, for every one-unit increase in
a-defensin, the odds of having active dental caries decreased by
25% (OR = 0.75, P = 0.004).
The fitted model shows that, while adjusting for age and gender;
and holding a-defensin, salivary total protein, salivary S. mutans,
and lactobacilli levels at fixed values, for every one-unit increase
in salivary pH, the odds of having active dental caries decreased
by 89% (OR = 0.11, P = 0.018).
The fitted model shows that, while adjusting for age and gender;
and holding a-defensin, salivary pH, salivary total protein, and
lactobacilli levels at fixed values, the odds of having active dental
caries for children with salivary S. mutans levels higher than or
equal to 105 cfu/ml were 8.8 times higher than children with salivary
S. mutans levels less than 105 cfu/ml (OR = 8.8, P = 0.001).
The fitted model shows that, while adjusting for age and gender;
and holding a-defensin, salivary pH, salivary pH, salivary total
protein, and S. mutans levels at fixed values, the odds of having
active dental caries for children with salivary lactobacilli levels
higher than or equal to 105 cfu/ml were 22 times higher than
children with salivary lactobacilli levels less than 105 cfu/ml (OR
= 22, p=0.001).
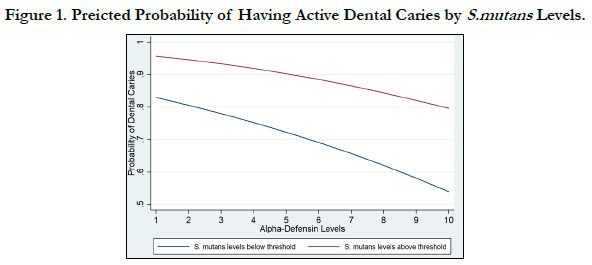
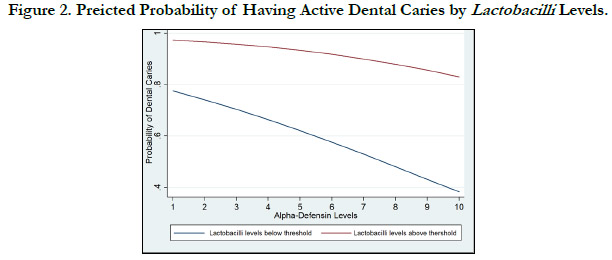
Figure 1 shows the visual representation of the probability of
having dental decay as a function of a-defensin and stratified by
S. mutans levels. Furthermore, figure 2 shows the visual representation
of the probability of having dental decay as a function of
a-defensinand stratified by lactobacilli levels.
Table 4. Results from multivariable logistic regression with active dental caries as dependent variable adjusted for age and gender.
Discussion
Saliva sampling is not an invasive method to indicate different
diseases and contain different microorganisms along with host
biological elements, which can be utilized for caries risk appraisal.
Presently, managing dental caries is directed toward comprehensive
dentistry, including preventive and minimally invasive approaches.
Caries risk assessment allows for early detection of risk
factors behind the dental caries process, the estimation of caries
incidence, and the likelihood of the changes in the activity of
carious decays.[19]
The current results indicated that salivary pH could be used as a
caries risk assessment indicator. The odds of having active dental
caries decreased significantly with the increased pH (Table 1).
These results confirm the results of other studies [20, 21] that
reported that caries-active individuals had higher amounts and faster acid production rates than caries-free individuals.[20]Also,
the mean levels of salivary pH were significantly decreased in caries-
active children compared to caries-free controls.[21]
The present study results showed that for every one-unit increase
in a-defensin, the odds of having active dental caries decreased
significantly (Table 1). These results confirm the previous results,
which indicated an inverse correlation between salivary a-defensin
and dental caries.[10, 11, 22]
The present study confirms the role of cariogenic microorganisms
(S. mutans and lactobacilli) in the dental caries process, suggesting
more dental caries in participants with more than 105
cfu/ml of cariogenic microorganisms (Table 1). These findings
confirmed the results of the previous studies.[23-26] The risk
assessment prediction is stronger for lactobacilli as participants
with salivary lactobacilli levels 105 cfu/ml or more were 22 times
higher than children with salivary lactobacilli levels lower than 105 cfu/ml (OR=22 for lactobacilli compared to 8.8 for S. mutans).
These results demonstrated that appropriate microbiological test
systems could compromise a ground work for improving the clinical
dental caries risk assessment. [27]
In comparison between caries affected compared to caries-free
participants, the salivary total protein concentration was higher in
caries-affected individuals. For every one-unit increase in salivary
total protein, the odds of having active dental caries increased
by 211% (OR = 2.11, P = 0.072). This increase in odd ratio was
not significant and disagreed with previous results. The present
results confirm the findings of other studies, which showed no
consistent relationship between salivary proteins and dental caries.[
28, 29] The lack of association between salivary proteins and
dental caries may be due to different protein levels with different
structures and functions.[15]
In this study, total antioxidant concentration was not associated
with dental caries, and these results were similar to the results of
other studies.[30, 31] The present results were agreed with that
of Ahmadi-Motamayel et al [32], who reported that Salivary and
serum TAC levels in caries affected and caries-free groups did not
show any significant differences.
Conclusion
In conclusion, a-defensin, salivary pH, and concentration of
cariogenic bacteria, especially lactobacilli, can be used as a caries
risk assessment marker. These results suggest new tools used for
screening and assessing caries vulnerability.
Acknowledgments
Many thanks to the dental records unit at Umm Al-Qura University,
Faculty of Dentistry and Dr. Wahdan Elkwatehy.
References
-
[1]. Loesche WJ. Role of Streptococcus mutans in human dental decay.Microbiol
Rev. 1986 Dec;50(4):353-80. PubMed PMID: 3540569.
[2]. Reich E, Lussi A, Newbrun E. Caries-risk assessment. Int Dent J. 1999 Feb;49(1):15-26. PubMed PMID: 10887469.
[3]. Hicks J, Garcia-Godoy F, Flaitz C. Biological factors in dental caries: role of saliva and dental plaque in the dynamic process of demineralization and remineralization (part 1). J ClinPediatr Dent. 2003 Fall;28(1):47-52. Pub- Med PMID: 14604142.
[4]. Van NieuwAmerongen A, Bolscher JG, Veerman EC. Salivary proteins: protective and diagnostic value in cariology? Caries Res. 2004 May- Jun;38(3):247-53. PubMed PMID: 15153696.
[5]. Devarajan H, Today SS-DI. Salivary proteins and its effects on dental caries– A review.Drug Invention Today. 2019;11(6):1406–1411.
[6]. Ganz T, Selsted ME, Szklarek D, Harwig SS, Daher K, Bainton DF, Lehrer RI. Defensins.Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985 Oct;76(4):1427-35. PubMed PMID: 2997278.
[7]. McKay MS, Olson E, Hesla MA, Panyutich A, Ganz T, Perkins S, Rossomando EF. Immunomagnetic recovery of human neutrophil defensins from the human gingival crevice.Oral MicrobiolImmunol. 1999 Jun;14(3):190- 3. PubMed PMID: 10495714.
[8]. Dale BA, Fredericks LP. Antimicrobial peptides in the oral environment: expression and function in health and disease. Curr Issues Mol Biol. 2005 Jul;7(2):119-33. PubMed PMID: 16053246.
[9]. Goebel C, Mackay LG, Vickers ER, Mather LE. Determination of defensin HNP-1, HNP-2, and HNP-3 in human saliva by using LC/MS. Peptides. 2000 Jun;21(6):757-65. PubMed PMID: 10958994.
[10]. Tao R, Jurevic RJ, Coulton KK, Tsutsui MT, Roberts MC, Kimball JR, Wells N, Berndt J, Dale BA. Salivary antimicrobial peptide expression and dental caries experience in children. Antimicrob Agents Chemother. 2005 Sep;49(9):3883-8. PubMed PMID: 16127066.
[11]. Dale BA, Tao R, Kimball JR, Jurevic RJ. Oral antimicrobial peptides and biological control of caries.BMC Oral Health. 2006 Jun 15;6Suppl 1(Suppl 1):S13. PubMed PMID: 16934114.
[12]. Klotman ME, Chang TL. Defensins in innate antiviral immunity.Nat Rev Immunol. 2006 Jun;6(6):447-56. PubMed PMID: 16724099.
[13]. Gardner MS, Rowland MD, Siu AY, Bundy JL, Wagener DK, Stephenson JL. Comprehensive defensin assay for saliva. Anal Chem. 2009 Jan 15;81(2):557-66. PubMed PMID: 19072583.
[14]. El-kwatehy W, Youssef A. Salivary biomarkers in caries affected and caries free children. International journal of dentistry and oral science. 2016;3 10): 48–352.
[15]. Toomarian L, Sattari M, Hashemi N, Tadayon N, AkbarzadehBaghban A. Comparison of neutrophil apoptosis, a-defensins and calprotectin in children with and without severe early childhood caries. Iran J Immunol.2011 Mar;8(1):11-9. PubMed PMID: 21427491.
[16]. Chiappin S, Antonelli G, Gatti R, De Palo EF. Saliva specimen: a new laboratory tool for diagnostic and basic investigation. ClinChimActa. 2007 Aug;383(1-2):30-40. PubMed PMID: 17512510.
[17]. Moritsuka M, Kitasako Y, Burrow MF, Ikeda M, Tagami J, Nomura S. Quantitative assessment for stimulated saliva flow rate and buffering capacity in relation to different ages. J Dent. 2006 Oct;34(9):716-20. PubMed PMID: 16504365.
[18]. Hsieh FY, Bloch DA, Larsen MD. A simple method of sample size calculation for linear and logistic regression. Stat Med. 1998 Jul 30;17(14):1623- 34. PubMed PMID: 9699234.
[19]. Fontana M, Zero DT. Assessing patients' caries risk. J Am Dent Assoc. 2006 Sep;137(9):1231-9. PubMed PMID: 16946427.
[20]. Animireddy D, Reddy Bekkem VT, Vallala P, Kotha SB, Ankireddy S, Mohammad N. Evaluation of pH, buffering capacity, viscosity and flow rate levels of saliva in caries-free, minimal caries and nursing caries children: An in vivo study. ContempClin Dent. 2014 Jul;5(3):324-8. PubMed PMID: 25191067.
[21]. Pyati SA, Naveen Kumar R, Kumar V, Praveen Kumar NH, Parveen Reddy KM. Salivary Flow Rate, pH, Buffering Capacity, Total Protein, Oxidative Stress and Antioxidant Capacity in Children with and without Dental Caries. J ClinPediatr Dent. 2018;42(6):445-449. PubMed PMID: 30085875.
[22]. Ribeiro TR, Dria KJ, de Carvalho CB, Monteiro AJ, Fonteles MC, de MoraesCarvalho K, Fonteles CS. Salivary peptide profile and its association with early childhood caries. Int J Paediatr Dent. 2013 May;23(3):225-34. PubMed PMID: 22892037.
[23]. Köhler B, Bjarnason S. Mutans streptococci, lactobacilli and caries prevalence in 15 to 16-year olds in Göteborg. Part II. Swed Dent J. 1992;16(6):253-9. PubMed PMID: 1481133.
[24]. Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology (Reading). 2003 Feb;149(Pt 2):279-294. PubMed PMID: 12624191.
[25]. Aas JA, Griffen AL, Dardis SR, Lee AM, Olsen I, Dewhirst FE, Leys EJ, Paster BJ. Bacteria of dental caries in primary and permanent teeth in children and young adults.J ClinMicrobiol. 2008 Apr;46(4):1407-17. PubMed PMID: 18216213.
[26]. Parisotto TM, Steiner-Oliveira C, Silva CM, Rodrigues LK, Nobre-dos- Santos M. Early childhood caries and mutans streptococci: a systematic review. Oral Health Prev Dent. 2010;8(1):59-70. PubMed PMID: 20480056.
[27]. Goeke JE, Kist S, Schubert S, Hickel R, Huth KC, Kollmuss M. Sensitivity of caries pathogens to antimicrobial peptides related to caries risk.Clin Oral Investig. 2018 Sep;22(7):2519-2525. PubMed PMID: 29372443.
[28]. Rudney JD, Staikov RK, Johnson JD. Potential biomarkers of human salivary function: a modified proteomic approach. Arch Oral Biol. 2009 Jan;54(1):91-100. PubMed PMID: 18804197.
[29]. Martins C, Buczynski AK, Maia LC, Siqueira WL, Castro GF. Salivary proteins as a biomarker for dental caries--a systematic review. J Dent. 2013 Jan;41(1):2-8. PubMed PMID: 23142096.
[30]. Dodwad R, Betigeri AV, Preeti BP. Estimation of total antioxidant capacity levels in saliva of caries-free and caries-active children. ContempClin Dent. 2011 Jan;2(1):17-20. PubMed PMID: 22114448.
[31]. Kumar D, Pandey RK, Agrawal D, Agrawal D. An estimation and evaluation of total antioxidant capacity of saliva in children with severe early childhood caries.Int J Paediatr Dent. 2011 Nov;21(6):459-64. PubMed PMID: 21718374.
[32]. Ahmadi-Motamayel F, Goodarzi MT, Mahdavinezhad A, Jamshidi Z, Darvishi M. Salivary and Serum Antioxidant and Oxidative Stress Markers in Dental Caries. Caries Res. 2018;52(6):565-569. PubMed PMID: 29698949.