Association of Tumor Necrosis Factor-a Gene Promoter Polymorphisms with Periodontitisin Type II Diabetic Syrian Population
Faten Kafa1*, Ali Abou Sulaiman2, Shaden Haddad1
1 Biochemistry and Microbiology Department, Faculty of Pharmacy, Damascus University, Syria.
2 Periodontology Department, Faculty of Dentistry, Damascus University, Syria.
*Corresponding Author
Faten Kafa,
Biochemistry and Microbiology Department, Faculty of Pharmacy, Damascus University, Syria.
E-mail: fatenkafa@yahoo.com
Received: September 19, 2021; Accepted: November 17, 2021; Published: November 20, 2021
Citation: Faten Kafa, Ali Abou Sulaiman, Shaden Haddad. Association of Tumor Necrosis Factor-a Gene Promoter Polymorphisms with Periodontitisin Type II Diabetic Syrian Population. Int J Dentistry Oral Sci. 2021;8(11):5064-5069. doi: dx.doi.org/10.19070/2377-8075-210001020
Copyright: Faten Kafa�2021. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Objectives: Tumor necrosis factor-a (TNF-a) is a proinflammatory cytokine that can regulate periodontal tissue health and
insulin-sensitive glucose uptake. TNF-a gene polymorphisms -857C/T and -1031T/C could influence periodontitisand type
II diabetes mellitus (DM). The goal of this studyis to evaluate the association between TNF-a gene polymorphisms (-857C/T
and -1031T/C) and both diseases in the Syrian population.
Design: 180 subjects were recruited and allocated into four groups: H= healthy control, DM= diabetes mellitus, ChP=
chronic periodontitis and ChPDM= chronic periodontitiswith DM.TNF-a SNPswereanalyzed by restriction fragment length
polymorphism (RFLP-PCR) technique.
Results: 857C/T gene polymorphism showed a lack of association with susceptibility to ChP, and T allele frequency was significantly
higher in DM (adjusted OR 3.116, 95% CI: 1.184- 8.2, p = 0.019), Whereas, -1031CC genotype hadmore frequency
in ChPDM group,and C allele has the higher probability in both diseases (OR= 3.013,CI: 1.275- 7.117, P = 0.011).
Conclusion: Thisstudy data suggested that the TNF-a polymorphism -1031T/C couldbe a potentialrisk factors of periodontitis,
but there were no association between -857C/T and periodontitis in diabetic patients in Syrian population.
2.Introduction
3.Materials and Methods
3.Results
4.Discussion
5.Conclusion
5.References
Keywords
Periodontitis; Diabetes Mellitus Type II; TNF-a; Promoter; Polymorphism.
Abbreviations
TNF-a: Tumor necrosis factor-a; DM: Type II Diabetes Mellitus; ChP: Chronic Periodontitis; ChPDM:
Chronic Periodontitis With Diabetes Mellitus; SNP: Single Nucleotide Polymorphism; MHC: Major Histocompatibility Complex;
CAL: Clinical Attachment Loss; PD: Pocket Depth; BMI: Body Mass Index; HbA1c: Glycated Hemoglobin; RFLP-PCR:
Restriction Fragment Length Polymorphism- Polymerase Chain Reaction; OR: Odds Ratio; CI: Confidence Interval; HWE:
Hardy-Weinberg Equilibrium; FFA: Free Fatty Acid.
Introduction
Periodontitis is acomplex inflammatory disorder caused by a
gram-negative bacterialplaque accumulated on the surfaces of
gingiva and teeth. It is induced by immune imbalance between the
plaque and host response [1]. The chronic type of periodontitis
(ChP) affects up to 50% of individuals [2]. ChP is defined bybacterial
plaque, inflammatory gingiva, formation of periodontal
pocket, loss of attachment and alveolar remodeling [3, 4].
Numerous risk factors can impede the immune balance [5], like
environmental factors and bad lifestyle habits, systemic diseases
such as diabetes, and genetic variables [1, 6, 7].
Diabetes mellitus Type 2 (DM) is a multifactorial metabolic diseaseincluding
hyperglycemia and modifying lipid metabolism. It
is caused by inadequate insulin secretion from �-islets and insulin
resistance [8]. Chronic hyperglycemia affects bone formation by
tissue oxidative stress [9], and increases the risk of periodontitis
in uncontrolled DM subjects [10].
Studies have shown common gene variations between DM and
periodontitis. However, this relationship needs more research
about the genes that may affect both diseases [11].
Cytokine gene Single Nucleotide Polymorphisms (SNPs) have
a crucial role in periodontal health regulation. Tumor necrosis factor-a (TNF-a) is one of potential proinflammatory cytokines
secreted mainly by macrophages.It induces the tissue injury and
bone resorption and many inflammatory pathways. It is involved
in phagocytosis, polymorphonuclear leukocytes (PMNs)- endothelial
cells adhesion, necrosis and apoptosis[12, 13]. Many
SNPswithin TNF-a gene associatewithperiodontitis, also can lead
to DM by stimulating insulin resistance [14].
The TNF-a gene consists of four exons and three introns located
in chromosome 6, short (p) arm, in the major histocompatibility
complex (MHC) class III region [15, 16].
Many researches showed that TNF-a expression and its activity
are affected by gene variables, which may increase the risk and
severity of periodontitis [17].
An Indian study has reportedthat the distribution difference of
-857C/T and -1031T/C polymorphisms was significantin periodontitis
subjects comparing to healthy group [17]. On the other
hand,-857T and -1031C alleleswere more frequent in periodontitis
groups in Japanese population [18]. Similarly, T allele in -857
polymorphism has significantly enhanced transcriptional activity
vs. C allele and tend to be more insulin resistant in an another
Japanese study of type 2 diabetic subjects [19].
Many studies have shown that -1031TT genotype might be an
important protective factor for ChP Asians. TNF expression by
mutant -1031CC genotype was significantly higher than -1031TT
genotype. Also,TNF protein secretion could be stimulated by
-1031T/C, and elevated cytokine levels may control periodontitis
progression [20].
Traditionally, many studies have investigated the association
of polymorphisms at -857C/T and -1031T/C positions with
ChP. Nonetheless, studies that examined these polymorphisms
in chronic periodontitis with diabetes mellitus (ChPDM) were
scarce.
Therefore, the aim of this study is to examine the potential role
of TNF-a gene polymorphisms at -857C/T and -1031T/C positions
in the susceptibility to ChP and DM type II in the Syrian
population.
Materials and Methods
Ethics statements
This study was approved by the Institutional Ethics Committee
at Damascus University, and it was conducted in accordance with
the Helsinki Declaration. All patients gave their consent about the
study purpose and nature.
Study population
The study is cross sectional enrolled100 Syrian participantsdiagnosed
ChP attending the Periodontology Department of Faculty
of Dentistry - Damascus University, 60 of them were type 2 diabetic
(ChPDM). Additionally, 40 race/ age-matched healthy subjects
(H), and 40 type 2 diabetics(DM) with healthy periodontal
tissues were recruited.
Inclusion criteria
Individualsaged 40 - 70 years, have a minimum of 20 teeth(other
than third molars), clinical attachment loss (CAL) = 3 mm in
two interproximal sites, and pocket depth PD =4 mm in =2
interproximal sites (on different tooth) [21], and body mass index
(BMI) between 19 - 30.5 kg/m2. Periodontitis severity was classified
depending on the pocket depth (PD) into three degrees:
primary (PD=4 � 5mm), moderate (6-7mm), severe (>7mm). The
type II DM participants were with glycated hemoglobin (HbA1c)
= 7%.
Exclusion criteria
Systemic diseasesthat may affect the immune responses, treatment
with antibiotics and/or NSAIDs last 3 months, pregnancy
and/ or breast feeding, smokers and alcoholics.
Sample Collection
From each subject, 3 ml of peripheral blood was collected in
EDTA tubes. Genomic DNA wasisolatedby a manual protocol
using urea and proteinase K [22]. DNA was quantified by measuring
260nm-absorbance using Nanodrop(Thermo scientific�,
USA), and then stored at -20�C until use.
Genotype determination
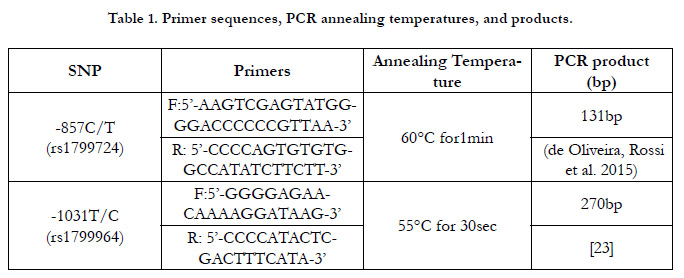
The TNF-aSNPs were genotyped using polymerase chain reaction
(PCR) and primers with sequences shown in Table (1). Then
PCR products were purified to remove excess salts and electrophorized
on agarose gel 2%. According to the digestive results
by HincII (-857T mutant allele)and BbsI(-1031C mutant allele)
enzymes(Jena Bioscience�, Germany), subjects were classified as
(TT) homozygotes, (CT) heterozygotes, or (CC) homozygotes.
Statistical analysis
SPSS Statistical software, version 25 (IBM�, New York, USA)
and GraphPad prism (version 9.1.2) were used to process the collected
data. Goodness-of-fit was analyzed by Hardy-Weinberg
equilibrium. The Chi-squared test was used to determine any
association between alleles or genotypes among the four study
groups. The effect degree was explicated as an odds ratio (OR)
with a 95% confidence interval (CI), and data was considered significant
at P value < 0.05.
Results
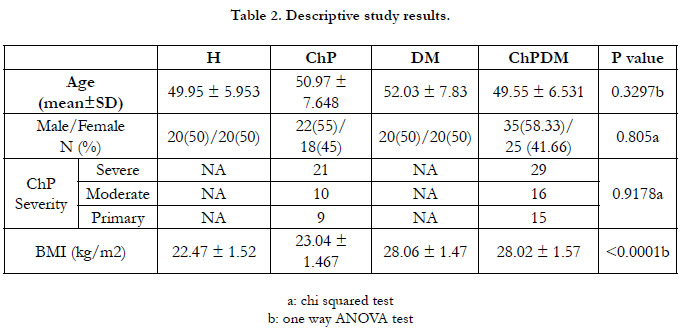
Study Groups were age and gender matched. Demographic data
are presented in Table (2), which also shows the distribution of
ChP and ChPDMpatients according to sex and disease severity
without any significant statistical differences between the study
groups (P<0.05), while differences with BMI values were significant
(P<0.001). Study Groups were age and gender matched.
Demographic data are presented in Table (2), which also shows
the distribution of ChP and ChPDMpatients according to sex
and disease severity without any significant statistical differences
between the study groups (P<0.05), while differences with BMI
values were significant (P<0.001).
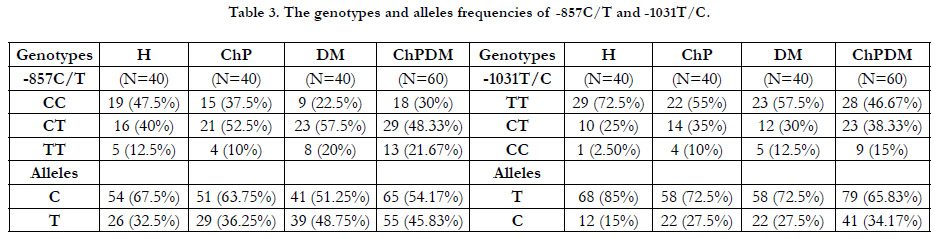
The genotype and allele frequencieswere in compatible with Hardy�
Weinberg Equilibrium (HWE), and there was no significant
differences between observed and expected genotypes in eachsubject
group (P= 1.99 ).
Genotypes and alleles frequencies of both SNPs were calculatedamong
groups, and shown in the Table (3). By using Chi squared
test, the associations between polymorphisms and susciptibility
to the diseases (periodontitis and DM) has been tested: H group
versus each group (ChP, DM and ChPDM). The effect degree
was expressed as OR with 95% CI.
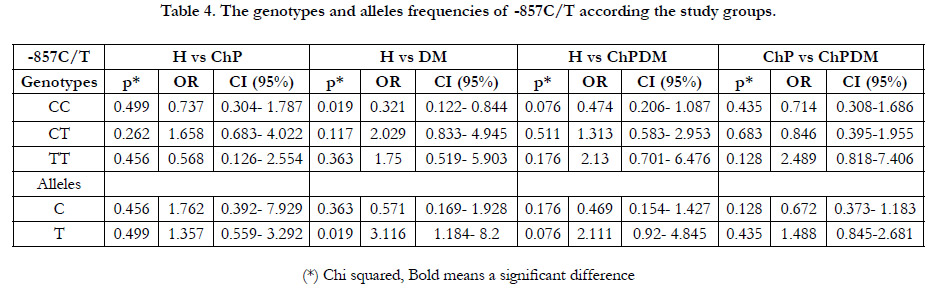
In Table (4), the -857C/T SNP in the TNF-agene promoter was
compared between H group and each other three groups of patients,
and showed no significant differences with ChP patients
with and without DM. However, DM subjects carrying the T allele
hadsignificantly an over 3-fold risk to develop the disease only
without ChP compared with C subjects (adjusted OR 3.116, 95%
CI: 1.184- 8.2, P = 0.019), and CC genotype had an important
protective role against diabetes mellitus (adjusted OR 0.321, 95%
CI: 0.122- 0.844, P = 0.019).
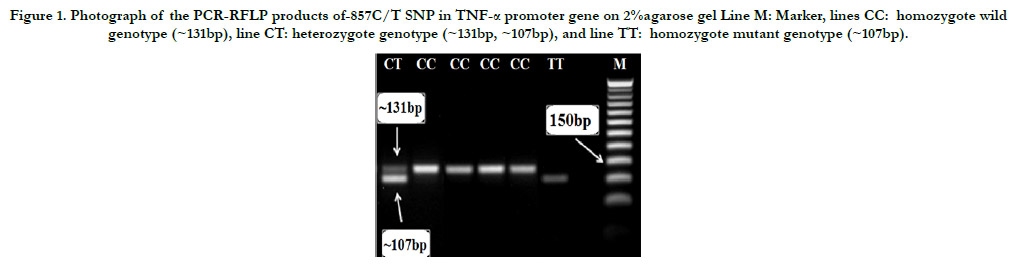
Restriction products of HincII restriction enzyme on 2% gel electrophoresis
are shown in Figure (1).
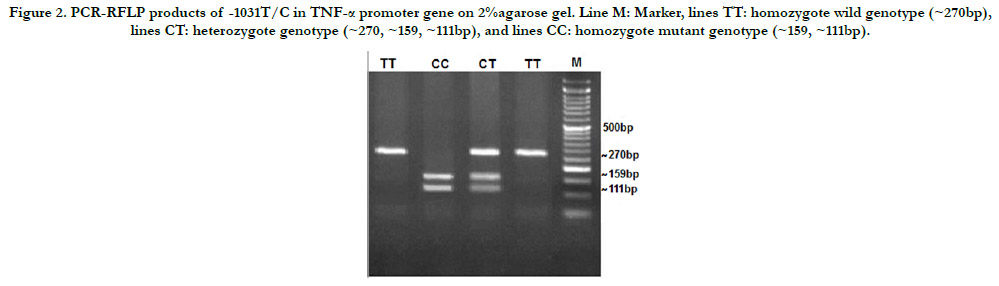
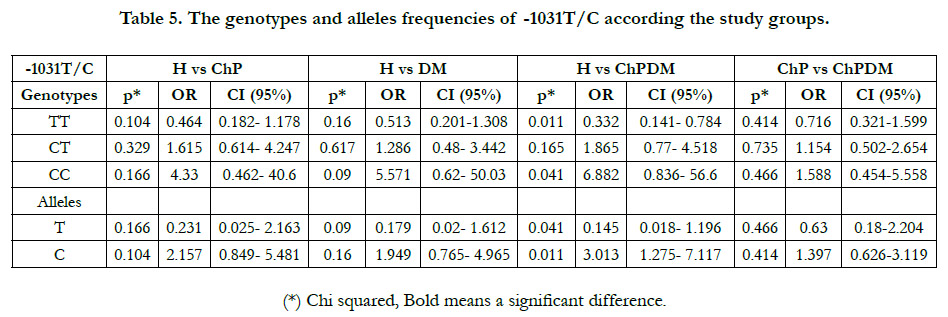
BbsI enzyme restriction fragments are electrophoresed on agarose
gel 2% (Figure 2), and it was confirmed in the Table (5) that
subjects with -1031CC genotype had almost 7-fold risk to develop
periodontitis together with DM (OR= 6.882, 95% CI: 0.836- 56.6,
P = 0.041), while there were not a significant difference between
healthy group and each disease alone.
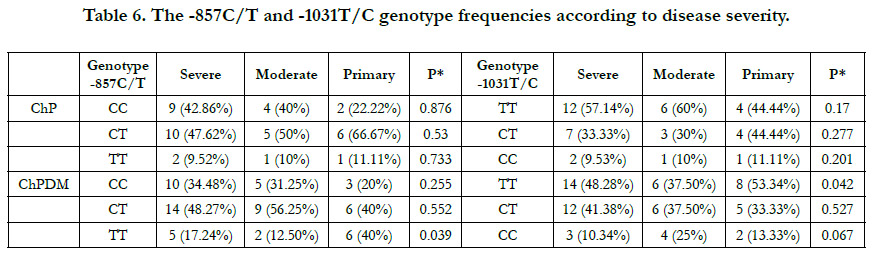
Then the frequencies of genotypes were calculated according
to disease severity, that the severity was divided to three groups:
severe, moderate, and primary. In -857C/T SNP, there were no
significant differences between these frequencies and groups of
severity (p>0.05) in ChP group, while the difference was significant
between TT genotype and severity in ChPDM subjects, that
this genotype is exist in primary subjects in significantly higher
percentage(p= 0.039) Table (6).
Also, in -1031T/C SNP, there was a significant difference between
severity and frequencies of wild genotype TT in ChP subjects
with DM,that it may have a protective role against periodontitis progression (p = 0.042).
Figure 1. Photograph of the PCR-RFLP products of-857C/T SNP in TNF-a promoter gene on 2%agarose gel Line M: Marker, lines CC: homozygote wild genotype (~131bp), line CT: heterozygote genotype (~131bp, ~107bp), and line TT: homozygote mutant genotype (~107bp).
Figure 2. PCR-RFLP products of -1031T/C in TNF-a promoter gene on 2%agarose gel. Line M: Marker, lines TT: homozygote wild genotype (~270bp), lines CT: heterozygote genotype (~270, ~159, ~111bp), and lines CC: homozygote mutant genotype (~159, ~111bp).
Discussion
Periodontitis is a multifacetedinfection triggered by microbial
plaque, that induces TNF-a expression. TNF-a can affect the immune
responsesby alteration of vascular endothelial function, and
modifying the preservative equilibrium and endothelium permeability.
It is considered as a risk factor of systemic inflammation
diseases with vascular dysfunction, such as diabetes [24], as well
as other environmental and /or behavioral factors like mouth hygiene
[25].
Many studies concerning the association between TNF-a gene
SNPs and susceptibility to periodontitis are available, but the results
were conflicting [20].
In this study, we had an attempt to find genetic association between
TNF-a and periodontal disease with and without DM.Cytokine
transcription and production levels may be influenced by their
gene polymorphisms, which in turn may induce the susceptibility
or resistance to several diseases, so this study has involved two
polymorphisms in TNF-a gene promoter.
Studies investigating -857C/T and -1031T/C SNPs in both periodontitis
and DM are rare. Literature review revealed that this
study is the first in Syria that evaluates the association between
TNF gene promoter SNPs and susceptibility to ChP in diabetic
patients.
This study has shown a significant difference between the groups
in -857CC genotype frequenciesin DM patients, but not in both
diseases. The subjects carrying the T allele were significantly more
likely to develop DM only without periodontitis compared with
C subjects.
Also, at -1031T/C there was a significant differencein genotype
CC between healthy group and ChPDM group, and subjects with
risk allele C were more likely to develop periodontitis together
with DM.
These findings are in line with many other studies that also foundno
correlation between ChP subjects and both genotypes -857C/
T and -1031T/C compared to healthy group in Indian population
[17]. In Japanese populationT allele of -857C/T and -1031C allele
were more elevated in periodontitis groups [18]. Also in Japanese
with DM type II, the gene promoter of TNF with -857Tallele significantly
stimulated transcriptional activity more than the -857C
promoter. Patients with TNF-a -857T allele tend to be more insulin
resistant [19]. In Chinese and Asians, -1031CC genotype was
significantly higher in ChPgroup compared with H group, while
there were a low level of association of -857C/T polymorphism
with susceptibility to ChP [26-28]. These results didn't match with
those in South Indian population, that -1031T/C polymorphism
had no association with ChP susceptibility [29].
Several lines of evidence have showed that variants of -1031
and -857 alleles have been related to elevated TNF-a secretion
[12, 30], and actas a critical factor in susceptibility of DM and its
severity [18]. TNF-a inhibits adipose lipoprotein lipase activity,
and induces hepatic lipogenesis, leading to excess metabolism of
plasma lipids. It stimulates lipolysis and inhibits the uptake of free
fatty acid (FFA). High FFA production can cause gluconeogenesis
induction, and finally hyperglycemia [14].
TNF-a expression, as a proinflammatory cytokine, has a key role
in periodontitis development, and raises resorption degree of the
alveolar bone and periodontal cell proliferation [12].
Even though it is known that-857C/T SNP can directly affect
the transcription efficiency of TNF-a, its functional effects have
been contentious on TNF-a cellular level. Recently, van Heel et
al. study suggested that blood TNF-a stimulates lipopoly saccharide
production in -857CC genotypes more than mutant T-allele
carriers. The transcription factor OTC1 binds to (ATGAAGAC)
sequence in TNF-a promoter from _858 to _851 position, only
with T allele in -857 site, to inhibit the function of promoter [31].
-1031T/C polymorphism could be a therapeutic prediction of the
response to TNF-a blockers [32].
Jain, P. et al showed that ChP increases serum TNF-a levels diabetic
patients [33], so we are still working on finding the link between
-1031 and -857 SNPs and its salivary levels in our study
groups.
Within the limits of this study, it can be suggested that TNF-a
-1031T/C SNP raises the risk of periodontitis, while there were
no association between -857C/T and periodontitis in diabetic patients
in Syrian population.
Conclusion
The study has been funded by Damascus University.
References
-
[1]. Stabholz, A., W.A. Soskolne, and L. Shapira, Genetic and environmental
risk factors for chronic periodontitis and aggressive periodontitis. Periodontol
2000, 2010. 53: p. 138-53.
[2]. Nazir MA. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci (Qassim). 2017 Apr-Jun;11(2):72-80. PubMed PMID: 28539867.
[3]. Wiebe CB, Putnins EE. The periodontal disease classification system of the American Academy of Periodontology--an update. J Can Dent Assoc. 2000 Dec;66(11):594-7. PubMed PMID: 11253351.
[4]. Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999 Dec;4(1):1-6. doi: 10.1902/ annals.1999.4.1.1. PubMed PMID: 10863370.
[5]. El Jilani MM, Mohamed AA, Zeglam HB, Alhudiri IM, Ramadan AM, Saleh SS, Elkabir M, Amer IB, Ashammakhi N, Enattah NS. Association between vitamin D receptor gene polymorphisms and chronic periodontitis among Libyans. Libyan J Med. 2015 Jan;10(1):26771. PubMed PMID: 28349804.
[6]. Beukers NG, van der Heijden GJ, van Wijk AJ, Loos BG. Periodontitis is an independent risk indicator for atherosclerotic cardiovascular diseases among 60 174 participants in a large dental school in the Netherlands. J Epidemiol Community Health. 2017 Jan;71(1):37-42. doi: 10.1136/jech-2015- 206745. Epub 2016 Aug 8. PubMed PMID: 27502782.
[7]. Kafa F, A Abou Sulaiman, S Haddad. Association between vitamin D receptor foki polymorphism and chronic periodontitis in Syrian population. International Journal of Pharmaceutical Sciences and Research. 2019;10(3):1336-1341.
[8]. Wu YY, Xiao E, Graves DT. Diabetes mellitus related bone metabolism and periodontal disease. Int J Oral Sci. 2015 Jun 26;7(2):63-72. PubMed PMID: 25857702.
[9]. Abdellatif HM, Binshabaib MS, Shawky HA, ALHarthi SS. Association between Periodontitis and Genetic Polymorphisms in Interleukins among Patients with Diabetes Mellitus. Dent J (Basel). 2021 Apr 18;9(4):45. Pub- Med PMID: 33919509.
[10]. Sharma N, Joseph R, Arun R, Chandni R, Srinivas KL, Banerjee M. Cytokine gene polymorphism (interleukin-1� +3954, Interleukin-6 [-597/-174] and tumor necrosis factor-a -308) in chronic periodontitis with and without type 2 diabetes mellitus. Indian J Dent Res. 2014 May-Jun;25(3):375-80. Pub- Med PMID: 25098998.
[11]. Cirelli T, Nepomuceno R, Rios ACS, Orrico SRP, Cirelli JA, Theodoro LH, Barros SP, Scarel-Caminaga RM. Genetic polymorphisms in the Interleukins IL1B, IL4, and IL6 are associated with concomitant periodontitis and type 2 diabetes mellitus in Brazilian patients. J Periodontal Res. 2020 Dec;55(6):918-930. doi: 10.1111/jre.12784. Epub 2020 Jul 9. PubMed PMID: 32648256.
[12]. Majumder P, Thou K, Bhattacharya M, Nair V, Ghosh S, Dey SK. Association of tumor necrosis factor-a (TNF-a) gene promoter polymorphisms with aggressive and chronic periodontitis in the eastern Indian population. Biosci Rep. 2018 Jul 31;38(4):BSR20171212. PubMed PMID: 29449347.
[13]. Grover HS, Saini R, Bhardwaj P, Bhardwaj A. Cytokines and other inflammatory mediators in periodontal health and disease. Indian Journal of Oral Health and Research. 2016 Jan 1;2(1):12.
[14]. Ayelign B, Genetu M, Wondmagegn T, Adane G, Negash M, Berhane N. TNF-a (-308) Gene Polymorphism and Type 2 Diabetes Mellitus in Ethiopian Diabetes Patients. Diabetes Metab Syndr Obes. 2019 Nov 28;12:2453- 2459. PubMed PMID: 31819571.
[15]. Molvarec A, Jermendy A, Nagy B, Kov�cs M, V�rkonyi T, Hupuczi P, Proh�szka Z, Rig� J Jr. Association between tumor necrosis factor (TNF)- alpha G-308A gene polymorphism and preeclampsia complicated by severe fetal growth restriction. Clin Chim Acta. 2008 Jun;392(1-2):52-7. PubMed PMID: 18396154.
[16]. Salles AG, Antunes LAA, Carvalho PA, K�chler EC, Antunes LS. Association Between Apical Periodontitis and TNF-a -308 G>A Gene Polymorphism: A Systematic Review and Meta-Analysis. Braz Dent J. 2017 Sep- Oct;28(5):535-542. PubMed PMID: 29215675.
[17]. Majumder P, Thou K, Bhattacharya M, Nair V, Ghosh S, Dey SK. Association of tumor necrosis factor-a (TNF-a) gene promoter polymorphisms with aggressive and chronic periodontitis in the eastern Indian population. Biosci Rep. 2018 Jul 31;38(4):BSR20171212. PubMed PMID: 29449347.
[18]. Soga Y, Nishimura F, Ohyama H, Maeda H, Takashiba S, Murayama Y. Tumor necrosis factor-alpha gene (TNF-alpha) -1031/-863, -857 single-nucleotide polymorphisms (SNPs) are associated with severe adult periodontitis in Japanese. J Clin Periodontol. 2003 Jun;30(6):524-31. PubMed PMID: 12795791.
[19]. Ohara M, Maesawa C, Takebe N, Takahashi T, Yamashina M, Ono M, Matsui M, Sasai T, Honma H, Nagasawa K, Fujiwara F, Kajiwara T, Taneichi H, Takahashi K, Satoh J. Different susceptibility to insulin resistance and fatty liver depending on the combination of TNF-a C-857T and adiponectin G+276T gene polymorphisms in Japanese subjects with type 2 diabetes. Tohoku J Exp Med. 2012 Feb;226(2):161-9. PubMed PMID: 22327199.
[20]. Xu L, Liu C, Zheng Y, Huang Y, Zhong Y, Zhao Z, Ma N, Zhang Z, Zhang L. Association of TNF-a-308G/A, -238G/A, -863C/A, -1031T/C, -857C/T polymorphisms with periodontitis susceptibility: Evidence from a metaanalysis of 52 studies. Medicine (Baltimore). 2020 Sep 4;99(36):e21851. PubMed PMID: 32899013.
[21]. Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012 Dec;83(12):1449-54. PubMed PMID: 22420873; PMCID.
[22]. Chacon Cortes DF, Griffiths L. Methods for extracting genomic DNA from whole blood samples: current perspectives. Journal of Biorepository Science for Applied Medicine. 2014;2014(2):1-9.
[23]. Bonyadi M, Abdolmohammadi R, Jahanafrooz Z, Somy MH, Khoshbaten M. TNF-alpha gene polymorphisms in Iranian Azari Turkish patients with inflammatory bowel diseases. Saudi J Gastroenterol. 2014 Mar- Apr;20(2):108-12. PubMed PMID: 24705148.
[24]. Kumar G, Ponnaiyan D, Parthasarathy H, Tadepalli A, Veeramani S. Evaluation of Endocan and Tumor Necrosis Factor-a as Inflammatory Biomarkers in Type 2 Diabetes and Periodontal Disease. Genet Test Mol Biomarkers. 2020 Jul;24(7):431-435. PubMed PMID: 32513032.
[25]. Petrovic SM, Nikolic N, Toljic B, Arambasic-Jovanovic J, Milicic B, Milicic T, Jotic A, Vidakovic M, Milasin J, Pucar A. The association of tumor necrosis factor alpha, lymphotoxin alpha, tumor necrosis factor receptor 1 and tumor necrosis factor receptor 2 gene polymorphisms and serum levels with periodontitis and type 2 diabetes in Serbian population. Arch Oral Biol. 2020 Dec;120:104929. PubMed PMID: 33091664.
[26]. Yang W, Jia Y, Wu H. Four tumor necrosis factor alpha genes polymorphisms and periodontitis risk in a Chinese population. Hum Immunol. 2013 Dec;74(12):1684-7. PubbMed PMID: 23973890.
[27]. Heidari Z, Moudi B, Mahmoudzadeh-Sagheb H. Immunomodulatory factors gene polymorphisms in chronic periodontitis: an overview. BMC Oral Health. 2019 Feb 12;19(1):29. PubMed PMID: 30755190.
[28]. Xu L, Liu C, Zheng Y, Huang Y, Zhong Y, Zhao Z, Ma N, Zhang Z, Zhang L. Association of TNF-a-308G/A, -238G/A, -863C/A, -1031T/C, -857C/T polymorphisms with periodontitis susceptibility: Evidence from a metaanalysis of 52 studies. Medicine (Baltimore). 2020 Sep 4;99(36):e21851. PubMed PMID: 32899013.
[29]. Lavu V, Venkatesan V, Bhaskar LV, Priyanka V, Kumarasamy P, Durairaj Paul SF, Rao SR. Polymorphic Regions in Fc Gamma Receptor and Tumor Necrosis Factor-a Genes and Susceptibility to Chronic Periodontitis in a Cohort From South India. J Periodontol. 2016 Aug;87(8):914-22. PubMed PMID: 27063995.
[30]. Banerjee M, Saxena M. Genetic polymorphisms of cytokine genes in type 2 diabetes mellitus. World J Diabetes. 2014 Aug 15;5(4):493-504. PubMed PMID: 25126395.
[31]. Kang CP, Lee KW, Yoo DH, Kang C, Bae SC. The influence of a polymorphism at position -857 of the tumour necrosis factor alpha gene on clinical response to etanercept therapy in rheumatoid arthritis. Rheumatology (Oxford). 2005 Apr;44(4):547-52. PubMed PMID: 15695296.
[32]. Yang W, Jia Y, Wu H. Four tumor necrosis factor alpha genes polymorphisms and periodontitis risk in a Chinese population. Hum Immunol. 2013 Dec;74(12):1684-7. PubMed PMID: 23973890.
[33]. Jain P, Ved A, Dubey R, Singh N, Parihar AS, Maytreyee R. Comparative Evaluation of Serum Tumor Necrosis Factor a in Health and Chronic Periodontitis: A Case-Control Study. Contemp Clin Dent. 2020 Oct- Dec;11(4):342-349. PubMed PMID: 33850400.