Assessment Of Oral Submucous Fibrosis Using Ultrasound As An Adjunct To Clinical Evaluation
Dr. Krishna S. Kumar1*, Dr. Jayaprasad Anekar2, Dr. Raj Achikanam Chirakara3
1 Assistant Professor, Amrita School of Dentistry (Amrita Vishwavidyapeetham), AIMS Health care campus, Edappily, Kochi, Kerala(Current Affliation),
Post graduate student, Department of Oral Medicine and Radiology, KVG Dental College and Hospital, Sullia DK, Karnataka, India. (Affliation
where the work was primarily carried out).
2 Professor and Head, KVG Dental College and Hospital, Sullia DK, Karnataka, India.
3 Professor and Head, MAHE Institute of Dental Sciences and Hospital, Chalakkara, Pallor, Mahe, Puducherry, India.
*Corresponding Author
Dr. Krishna S. Kumar MDS,
Assistant Professor, Amrita School of Dentistry (Amrita Vishwavidyapeetham), AIMS Health care campus, Edappily, Kochi, Kerala(Current Affliation), Post graduate student,
Department of Oral Medicine and Radiology, KVG Dental College and Hospital, Sullia DK, Karnataka, India. (Affliation where the work was primarily carried out).
Tel: 8330875475
E-mail: krishna.santhosh32@gmail.com
Received: October 19, 2021; Accepted: November 10, 2021; Published: November 20, 2021
Citation: Dr. Krishna S. Kumar, Dr. Jayaprasad Anekar, Dr. Raj Achikanam Chirakara. Assessment Of Oral Submucous Fibrosis Using Ultrasound As An Adjunct To Clinical Evaluation. Int J Dentistry Oral Sci. 2021;8(11):5042-5048. doi: dx.doi.org/10.19070/2377-8075-210001016
Copyright: Dr. Krishna S. Kumar MDS©2021. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Objectives: To Evaluate oral tissues affected by Oral submucous fibrosis using Ultrasound Color Doppler. Comparative
evaluation of the clinical stages of Oral submucous fibrosiswith Ultrasound Color Doppler findings will be done thereby
assessing the severity of the disorder.
Methods and Material: The study included 39 case subjects, who were grouped into 3 stages based on clinical characteristics.
Control group had 40 healthy subjects from the same geographic population who were both age and sex matched. Both
the case and control group were subjected to ultrasound evaluation of buccal mucosa using a medical sonographic unit GE
VOLUSON 730 pro, Color Doppler Ultrasound system.Statisticalanalysis was done using ANOVA test and fisher’s exact test.
Results: In the control group all subjects demonstrated a hypoechoic submucosa which could be clearly differentiated from
the muscle layer. In the case group there was increased echogenicity compared to the control group. Submucosal thickness
increased and vascularity was decreased as the disease progressed to stage II and stage III. In advanced cases of oral submucous
fibrosis submucosa could not be differentiated from the muscle layer clearly.
Conclusions: Ultrasound Color Doppler examination can be a promising diagnostic tool for oral submucous fibrosis. It can
even pick up positive sites affected by the disease in initial stages which may not be evident in clinical examination alone and
hence can be used as an adjunctive investigation modality and for assessment of the severity of the disease, especiallywhere
doing biopsy is not feasible.
2.Introduction
3.Materials and Methods
3.Results
4.Discussion
5.Conclusion
5.References
Keywords
Buccal Mucosa; Color Doppler; Oral Submucous Fibrosis; Ultrasound.
Introduction
Oral submucous fibrosis (OSMF) is a common potentially (pre)
malignant lesion [1] affecting the oral mucosa which might extend
even into the pharynx, oesophagus or rarely into the larynx. It
is commonly seen affecting individuals of South Asian countries
especially among the Indian population where chewing of betel
quid with arecanut is a customary practice. Increasing use of commercially
manufactured products of betel quid which contain tobacco,
arecanut and other substances in powdered or granulated
form is also extremely alarming as it is associated with increased
risk of malignancy [2].
Clinically OSMF is characterized bypresence of fibrous bands,
blanching of mucosa and burning sensation especially on consumption
of spicy foods. Reduction inmouth opening and cheek
flexibility along with difficulty in tongue movements will be seen
as a result of the fibrosis of the oral tissues [3, 4]. Involvement
of the underlying submucosa and muscles may be present with
disease progression. There will be also obliteration of blood vessels
resulting in decreased vascularity and epithelial atrophy.Clinical
assessment is subjective due to observer variability and alone it
may be insufficient to assess the disease severity. Disease severity
will be different in different sites of the oral cavity and biopsy taken from a single site may not characterize the severity of the
entire disease. Hence the correlation between clinical and histological
grading of the disease is poor [2].
Ultrasound (US) examination is a real time imaging modality
which can provide both qualitative and quantitative assessment
of the disease [3]. Color Dopplerprovides color coded representation
of the perfused areas and gives information of vascularity
of different tissues [5]. Hence this study aims in the assessment
of the severity of OSMF using ultrasound and Color Doppler
and the comparative evaluation of the stages of OSMF with ultrasound
and color Doppler examination findings.
Materials and Methods
The study group consisted of 79 patients who visited the Department
of Oral Medicine and Radiology in a teaching dental
hospital, Sullia.
Patients were divided in to case and control groups (age and sex
matched). An ethical clearance certificate was obtained from the
institutional ethical committee to proceed with the study. Each
sample of the selected group was informed about the study in patients
known language and informed consent was obtained. The
control group (Group –1) consisted of 40 patients with a negative
history of chewing arecanut or its commercial preparations. Study
group (Group-2) consisted of 39 Patients who were clinically diagnosed
suffering from various stages of OSMF.
The exclusion criteria were:
Patients with known history of:
• Trismus owing to causes other than OSMF
• Previous biopsy or treatment for OSMF
• Any mucosal conditions where blood flow of the oral tissues are
affected which includes anaemia, oral cancer and other coexisting
oral mucosal lesions.
• Any systemic and skin diseases which includes scleroderma,
amyloidosis, diabetes mellitus and hypertension.
• Radiation therapy done in the head and neck region
Both the case and control group were subjected to sonographic
evaluation of buccal mucosa in the Department of Radiology,
KVG Medical College.
Detailed history was elicited from each subject of group-2 and
the data was entered into a structured Performa. The clinical examination
was carried out and patients were divided into 3 stages
with 13 patients in each group according to the classification proposedby
Bose and Balan [6].
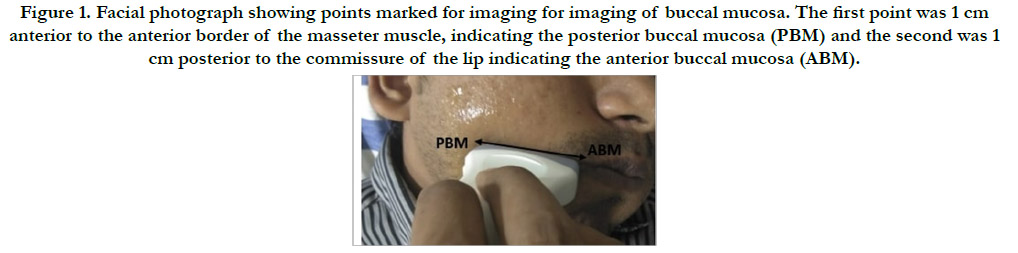
Two reference points were considered for ultrasonographic imaging
of buccal mucosa. The first reference point indicated was
posterior buccal mucosa (PBM) which was 1 cm anterior to the
anterior border of the masseter muscle. The second reference
point was anterior buccal mucosa (ABM) which was 1 cm posterior
to the commissure of the lip. An imaginary line was drawn
between ABM and PBM [7] (Figure 1). Palpation for the presence of fibrous bands was done in; Left anterior buccal mucosa (LAB),
Left posterior buccal mucosa (LPB), Right anterior buccal mucosa
(RAB), Right posterior buccal mucosa (RPB), for comparative
evaluation with the ultrasound findings [2].
Ultrasound evaluation
Ultrasound scan of the buccal mucosa bilaterally were performed
for all subjects using a medical sonographic unit GE VOLUSON
730 pro, Color Doppler Ultrasound system. A multifrequency linear
transducer with frequency range from 6-13MHz was used. To
avoid any bias related to the assessment of the echogenicity of
the tissues technical parameters of the scan were maintained same
during the entire study. Patient was made to lie in the supine position
for the scan and it was carried out for anterior and posterior
buccal mucosa separately on each side.
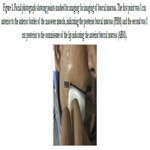
For imaging of the buccal mucosa transcutaneous extraoral transducer
was positioned onthe cheek along an imaginary line which
joins the inferior point of the tragus of the ear to the commissure
of the mouth. This line will be parallel to the lower border of the
mandible (Figure 2). Patient was asked to blow the cheek so as
to define the mucosal structures clearly [8]. For imaging of the
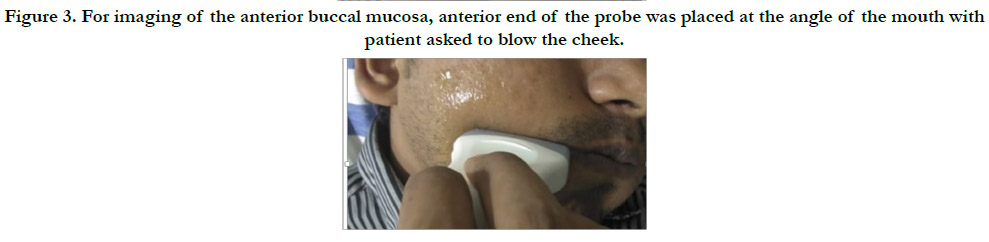
anterior buccal mucosa, anterior end of the probe was placed 1
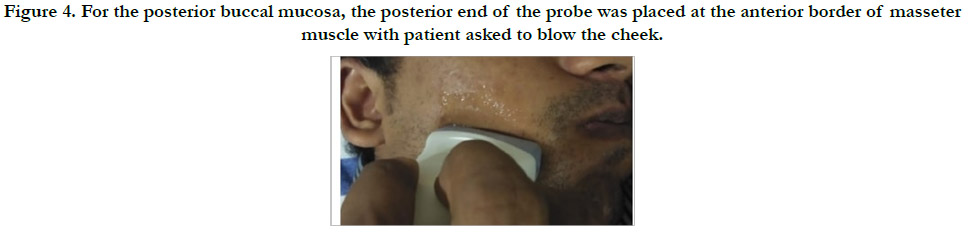
cm posterior to the commissure of the lip (Figure 3). For the posterior
buccal mucosa, the posterior end of the probe was placed
1 cm anterior tothe anterior border of masseter muscle (Figure
4). US findings were recorded separately for the right and left
anterior and posterior buccal mucosa. The echotexture of submucosa
and its differentiation from the underlying muscle layer was
recorded. The blood flow of the buccal mucosa was noted using
Color Doppler Ultrasound.
Irrespective of the study findings all the patients included in this
study underwent routine management for OSMF.
Results
The US appearance of the cross section of buccal mucosa in the
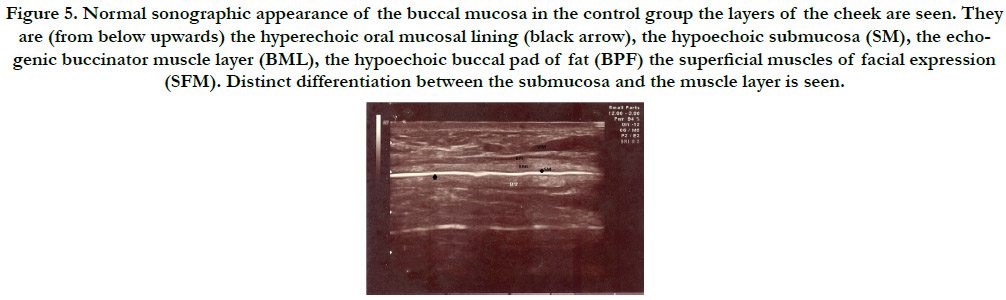
control group was as follows. The oral mucosal lining appeared as
a hyperechoic line (black arrow). Submucosa (SM) corresponds to
the hypoechoic zone supported by an echogenic muscle layer corresponding
to the buccinator muscle (BML). Submucosa could
be clearly differentiated from the muscle layer. Buccal pad of fat
(BPF) appears as a hypoechoic zone and muscles of facial expression
(SFM) can be also appreciated (Figure 5). This US appearance
of the layers of cheek in controls were similar to the normal
echo architecture of the cheek layers from skin to the mucosa as
reported in previous studies [2].
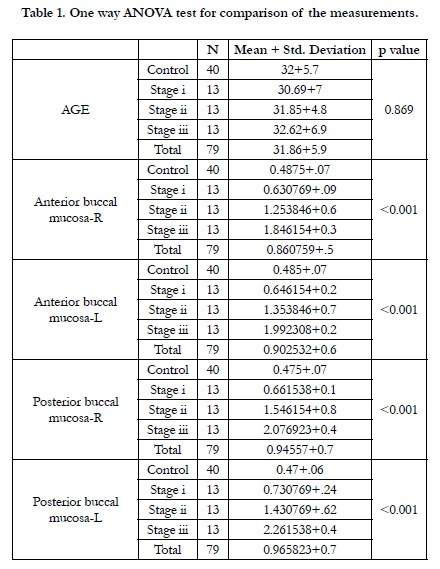
The obtained values of this study were subjected to statistical
analysis using SPSS software version 20. One way ANOVA TEST was used for the comparison of values of the case and control
group. The threshold for significance was set at p< 0.05. Out of
79 subjects, 66(83.5%) were males and 13(16.4%) were females.
Mean age of patients in group-1 was 32 years and group-2 was
31.7 years. Comparison of AGE using one way ANOVA test
showed that there was no statistical significance for the difference
in age between the control and case group. Comparison of
groups based on gender showed that maximum patients in all
the case groups were males. There were 4 females (30.8%) and 9
males (69.2%) in stage 1 group, 2 females (15.4%) and 11 males
(84.6%) in stage 2 group and 1 female (7.7%) and 12 males (92.3)
in stage 3 group. (Table 1)
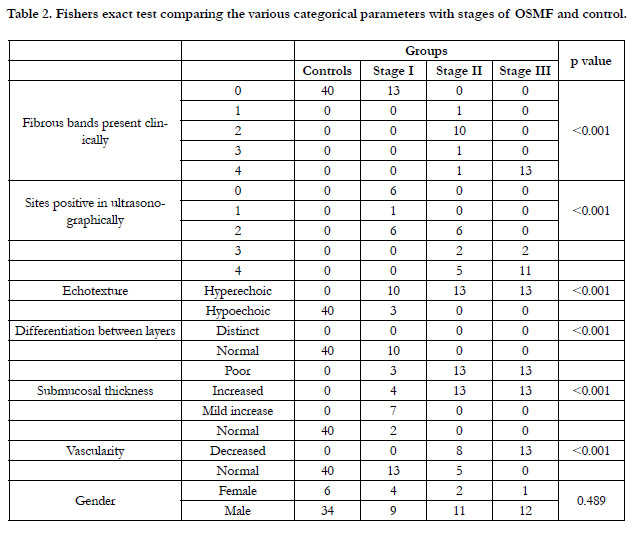
Comparison of case group and controls with the number of fibrous
bands present clinically and with the number of sites positive
in ultrasound examination showed that maximum number of
fibrous bands and sites positive was present in stage 3 OSMF
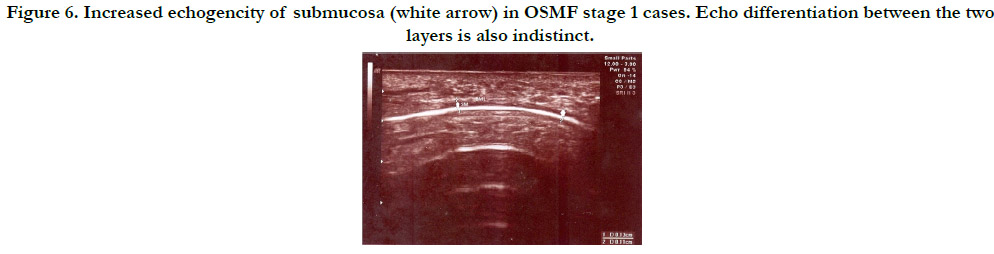
cases and minimum in control cases. Comparison of echotexture
seen in various groups showed that control group had hypoechoic
echotexture whereas in case group it appeared hyperechoic for
stage 2 and stage 3. In stage 1 case group, 10 patients (76.9%) had
hyperechoic areas and 3 patients (23.1%) had hypoechoic areas. In
the case group, all stage 2 and stage 3 cases showed hyperechoic
appearance. Clear differentiation between the submucosa and the
muscle layer was found in all patients of the control group. In
the case group it was not distinct and as the disease progressed it
was totally lost. In Stage 1 group of patients, in 10 cases (76.9%)
differentiation was distinct and in 3 cases (23.1%) it was poor. In
stage 2 and stage 3 group of patients there was total loss of differentiation.
(Table 2, Figure 6 and Figure 7).
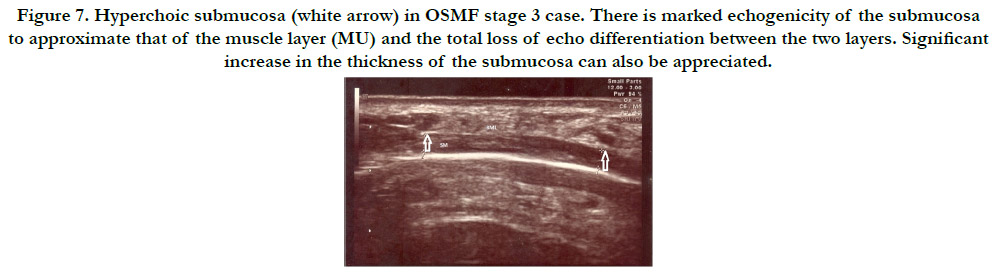
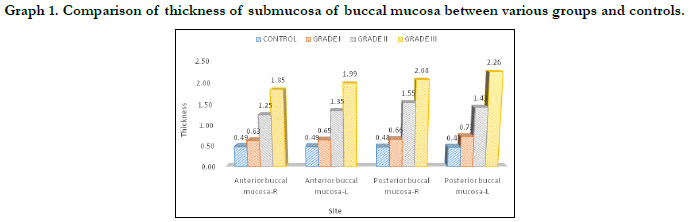
The mean submucosal thickness of controls was found to be
0.48mm. Submucosal thickness increased as the disease progressed
from stage 1 to stage 3 in the case group. (Figure 6 and
figure 7) In 2(15.4%) stage 1 cases, thickness of the submucosa
fell under the normal range while 7 cases (53.8%) had mild increase
and 4(30.8%) cases had increased thickness of submucosa.
Increase in the thickness of the submucosa was found to be more
in the posterior buccal mucosa compared to the anterior buccal
mucosa on both right and left side. (Table 1 and Graph 1)Post
Hoc Tukey analysis was done and it showed statisticallysignificant
difference between all the pairs (p value<0.05) except between the
control and grade 1 group (p>0.05).
On Color Doppler examination all group 1 controls (100%)
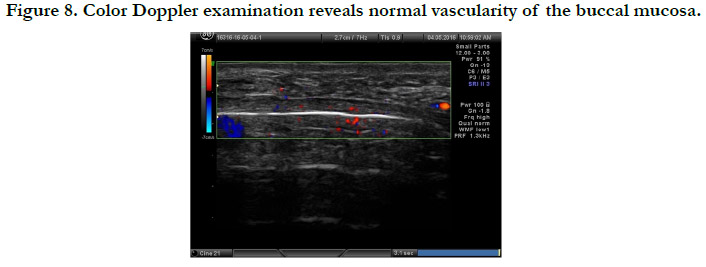
demonstrated normal vascularity of buccal mucosa.(Figure 8) In
group 2, stage 1 cases (100%) demonstrated normal vascularity.
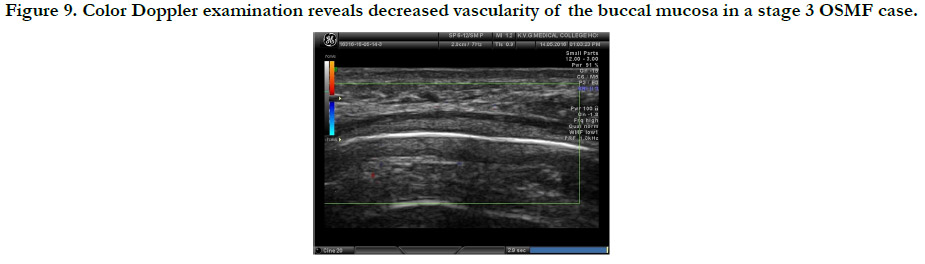
In stage 2 group, 8 cases (61.5%) showed decreased vascularity
and 5 cases showed normal vascularity, whereas all stage 3 OSMF
patients (100%) demonstrated decreased vascularity. (Figure 9)
This shows that there is decrease in vascularity as the disease progresses
to stage 3.(Table 2)
Figure 1. Facial photograph showing points marked for imaging for imaging of buccal mucosa. The first point was 1 cm anterior to the anterior border of the masseter muscle, indicating the posterior buccal mucosa (PBM) and the second was 1 cm posterior to the commissure of the lip indicating the anterior buccal mucosa (ABM).
Figure 2. Position of the transducer is along the line joining the angle of mouth to the inferior point of the tragus of ear parallel to the lower border of the mandible.
Figure 3. For imaging of the anterior buccal mucosa, anterior end of the probe was placed at the angle of the mouth with patient asked to blow the cheek.
Figure 4. For the posterior buccal mucosa, the posterior end of the probe was placed at the anterior border of masseter muscle with patient asked to blow the cheek.
Figure 5. Normal sonographic appearance of the buccal mucosa in the control group the layers of the cheek are seen. They are (from below upwards) the hyperechoic oral mucosal lining (black arrow), the hypoechoic submucosa (SM), the echogenic buccinator muscle layer (BML), the hypoechoic buccal pad of fat (BPF) the superficial muscles of facial expression (SFM). Distinct differentiation between the submucosa and the muscle layer is seen.
Figure 6. Increased echogencity of submucosa (white arrow) in OSMF stage 1 cases. Echo differentiation between the two layers is also indistinct.
Figure 7. Hyperchoic submucosa (white arrow) in OSMF stage 3 case. There is marked echogenicity of the submucosa to approximate that of the muscle layer (MU) and the total loss of echo differentiation between the two layers. Significant increase in the thickness of the submucosa can also be appreciated.
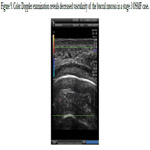
Figure 9. Color Doppler examination reveals decreased vascularity of the buccal mucosa in a stage 3 OSMF case.
Table 2. Fishers exact test comparing the various categorical parameters with stages of OSMF and control.
Discussion
OSMF is a chronic inflammatory potentially pre (malignant) disorder
where the disease severity varies from site to site. It has a
prevalence rate of 0.2 to 1.2% with a high malignant transformation
rate of 3-7.6% and is very common in Indian population. It
is incurable and irreversible even after the cessation of the habit
[3, 9]. It is characterized by progressive fibrosis and hence requires
periodic monitoring, assessment of response to habit cessation
and various medical and surgical treatment modalities. Multiple
regions of oral cavity may be affected because OSMF is a diffuse
disease. Hence even thoughbiopsy is considered as the final standard
for the diagnosis of OSMF, it may not be possible to perform
biopsy from multiple sites of the oral cavity for histopathological
evaluation. And, biopsy taken from a single site may not represent
the entire disease severity. Biopsy itself can induce scarring and
further limit the mouth opening [2].
A Cochrane systematic review done on interventions for the management
of OSMF pointed out that extraoral imaging may be
used to assess the disease severity with high sensitivity [10]. Ultrasound
is a safe, non-invasive, readily available, cost effective modality
which provides real time imaging of the tissues being examined.
Evidence for adverse biological effects of ultrasound used
in diagnostic range is not reported in literature [11]. Evaluation
of the tissues is done by assessing the difference in the thickness
and echogenicity of the mucosal structures. It has also proved
to be an efficient deep heating modality and if applied at higher
intensity for a longer duration can soften the fibrous tissues [12].
It has been used for diagnosis and follow up of systemic diseases
like scleroderma. Scleroderma is characterized by the presence
of fibrosis and chronic inflammation like OSMF, but they differ
in aetiology and clinical presentation which helps to differentiate
them [2, 3]. It was found out that areas with increased collagen
deposition have high elastic moduli and present with hyperechogencity
in ultrasound images [13]. Buccal mucosa is almost always
involved in OSMF. Easy ultrasonographic examination is possible
due to its superficial location and absence of underlying bone.
Presence of fibrous bands in the buccal mucosa is its most common
clinical presentation and in 4.5% of population, 90% of the
epithelium overlying it becomes atrophic and it is the common
site for malignant transformation [2].
As the tissue architecture will be relatively constant in the adult
population, patients falling under the age group of 18-40 years
was chosen for the study [2]. The age characteristics of the present
study group were in accordance with the mean age of OSMF
patients of 26.1 years as reported by the previous studies. The
majority of the present cases were in the age range of 21 - 30
years. Similar to the previously reported studies in this study also
majority of the patients in the case group were men. In Indian
society usage of commercial betel quid products is seen more
among men than women and that may be the reason for male
predominance in the present study also. This is alarming asit reflects
the changing lifestyle of young Indian men [14].
Patients with systemic disease and other mucosal lesions which
can result in fibrosis of the oral mucosa and alteration of vascularity
were excluded from the study population as it can produce
false positive results. Transcutaneous ultrasound examination was
preferred as it will be difficult to place an intraoral transducer in
patients with OSMF due to decreased interincisal mouth opening
[2].
No OSMF cases which demonstrated palpable fibrous bands in
clinical examination showed complete hypoechogenicity on US
examination. This suggests that all cases of OSMF detected clinicallycan
be also detected in ultrasound examination. It could even
detect positive findings in stage 1 cases where there were no clinically
palpable fibrous bands and hence it may be considered as an
initial investigation modality in cases diagnosed with OSMF as the
findings can affect the treatment protocol and prognosis of the
patient. As the disease progresses from stage 1 to 3submucosa
could not be distinguished from the underlying muscle layer due
to complete loss of echo differentiation between them. All these
findings support the study results of Krithika et al who reports
that complete loss of echo differentiation is highly indicative of
well-established oral submucous fibrosis [2].
In the present study the mean submucosal thickness of controls
was 0.48mm which fell under the normal range between 0.45mm-
0.56mm. There was a steady increase in submucosal thickness
from stage 1 to stage 3 cases in the case group. Rangaiah et al
[3] also reported that submucosal thickness will be increased in
OSMF patients, compared with controls which is similar to the
present study. This is also in accordance to the study conducted
by Agarwal et al, Tiwari et al and Devathambi and Aswath
where they found statistical correlation between the severity of
the disease and the submucosal thickness [4, 5, 7]. It was found
that there was an increased thickness of submucosa in posterior
buccal mucosa than the anterior buccal mucosa as most of the
patients tends to keep the betel quid in that region. As disease
progresses from stage 2 to stage 3, significant increase in the submucosal
thickness is noted. Nadendla et al in their study to assess
the severity of OSMF by correlation between the clinical staging
and sonographic findings found out that there is high correlation between the increase in submucosal thickness with the stage of
the disease [11].
In our study we could find significant correlation between the
OSMF stages and the findings from ultrasonography examination
in all the groups except between the control and stage I group
supporting the study done by Dupare et al [9].
Color Doppler examination can provide information about the
vascularity which plays a significant role in the assessment of the
outcome of the treatment modalities and prognosis of the disease
condition. Decreased vascularity indicates poor prognosis,
and more than average vascularity suggests malignant changes
because of angiogenesis [9]. In the present study vascularity was
found to be normal in stage 1 cases and is steadily decreasing
as the disease severity increases. This supports the findings of
study conducted by Manjunath et al who reports of decrease of
vascularity in color Doppler ultrasound as the disease progresses
[15]. Tiwari et al in a similar study reported of slight decrease in
vascularity in stage I cases compared to control [16].
In the present study hyperechogenicity of the submucosa was
present even in clinically negative sites on ultrasound examination.
There was good correlation between the increase in submucosal
thickness, loss of differentiation between the submucosa
and muscle layer and increase in echogenicity of the mucosa with
the stage of the disease. Hence Ultrasound Color Doppler examination
can be considered as an adjunct to clinical examination in
the assessment of the severity of OSMF and as an investigative
modality especially in cases where it is not feasible to do a biopsy.
Histological grading is considered as the gold standard for executing
the treatment plan of OSMF. In this study clinical staging of
the disease was compared to the findings in the ultrasonographic
examination, which is a potential limitation of the study. Future
studies may be done evaluating the correlation between clinical
and ultrasonography findings with histological grading.
Acknowledgement
We are very thankful to Dr. Rudresh Hiremath, Professor, Department
of Radiology K V G Medical College and Hospital, Sullia
and Dr. R Venkitachalam, Assistant Professor, Department of
Public Health Dentistry Amrita School of Dentistry, Kochi for all
the support and help provided.
References
-
[1]. van der Waal I. Historical perspective and nomenclature of potentially malignant
or potentially premalignant oral epithelial lesions with emphasis on
leukoplakia-some suggestions for modifications. Oral Surg Oral Med Oral
Pathol Oral Radiol. 2018;125:577–81.
[2]. Krithika C, Ramanathan S, Koteeswaran D, Sridhar C, Satheesh J, Shiva Shankar MP. Ultrasonographic evaluation of oral submucous fibrosis in habitual areca nut chewers. Dentomaxillofac Radiol. 2013;42(9):1-8.
[3]. Rangaiah P, Annigeri RG, Lingappa A. Transcutaneous ultrasonographic assessment of oral submucous fibrosis: A preliminary study. Int J Oral Med Sci.2010;9:137-47.
[4]. Agarwal RK, Hebbale M, Mhapuskar A, Tepan M. Correlation of ultrasonographic measurements, histopathological grading, and clinical staging in oral submucous fibrosis. Indian J Dent Res. 2017;28:476-81.Pubmed PMID: 29072206.
[5]. Tiwari M, Gupta M, Ghom S, Devi B. Ultrasonographic evaluation of oral submucous fibrosis: A preliminary study. International Journal of Women Dentists 2014;1(1):1-4.Pubmed PMID: 24516775.
[6]. More CB, Das S, Adalja C, Kamatchi V, Venkatesh R. Proposed clinical classification for oral submucous fibrosis. Oral Oncol. 2012;48:200-2.Pubmed PMID: 22070918.
[7]. Devathambi JR, Aswath N. Ultrasonographic evaluation of oral submucous fibrosis and masseteric hypertrophy. J Clin Imaging Sci. 2013;3 Suppl 1:12. Pubmed PMID: 24516775.
[8]. Bharat BM, Joshi JS, Patil SD. Triple Protocol Approach: A Modified Extended Approach of High Resolution Ultrasonography of cheek. Poster presented at: European Congress of Radiology 2011;Vienna, Australia.
[9]. Dupare A, Dhole A, Motwani M. Ultrasonographic evaluation of oral submucous fibrosis patients: A non-invasive diagnostic approach. J Indian Acad Oral Med Radiol. 2018;30:247-52.
[10]. Federowicz Z, Chan Shih-Yen E, Dorri M, Nasser M, Newton T, Shi L. Interventions for the management of oral submucous fibrosis. Cochrane Database Syst Rev .2009;1:CD007156.
[11]. Nadendla LK, Tatikonda VK, Bangi BB, Bhavya H, Devulapally RV, Pokala A. Sonographic imaging of fibrosis of oral mucosa and its correlation with clinical staging in oral submucous fibrosis. J Can Res Ther. 2018;14:394-7.
[12]. Dani VB, Patel SH. The effectiveness of therapeutic ultrasound in patients with oral submucosal fibrosis. Indian J Cancer 2018;55:248-50.Pubmed PMID: 30693888.
[13]. Thapasum FA, Rangdhol V, Mohammed F, Mohamed S, Shanmugam S. Gray-scale ultrasonographic imaging of the buccal mucosa in various stages of oral submucous fibrosis. Oral Radiol. 2014;31(3):143-8.
[14]. Reddy V, Wanjari PV, Bamda NR, Reddy P. Oral Submucous Fibrosis: Correlation of clinical grading to various habit factors. Int J Dent Clin. 2011;3:21-4.
[15]. Manjunath K, Rajaram PC, Saraswathi TR, Sivapathasundharam B, Sabarinath B, Koteeswaran D et al. Evaluation of oral submucous fibrosis using ultrasonographic technique: A new diagnostic tool. Indian J Dent Res. 2011;22:530-6.
[16]. Tiwari M, Deoghare A, Sharma A, Saha S, Poptani R. Evaluation of OSMF with ultrasonography. Int J Oral Health Dent. 2017;3(3):169-74.