Combined Use of Hyaluronic Acid with Nano-bioactive Glass Enhanced BiocementBased Silicate Stimulated Bone Regenerative Capacity in Tibial Bone Defects of Rabbits: In-Vivo Study
Fatema Aziz Al-Sayed1*, Dr. Radwa Hegazy2, Zeinab Amin3, Dr.Hanan El-Beherie4
1 Department of Oral Biology, Faculty of Dentistry, Cairo University, Cairo, 11553, Egypt.
2 Department of Oral Biology, Faculty of Dentistry, Cairo University, Cairo, 11553, Egypt.
3 Department ofOral Biology, Faculty of Dentistry, Cairo University, Cairo, 11553, Egypt and Oral Biology Department, Faculty of Dentistry, Ahram Canadian University, Giza, Egypt.
4 Department of Biomaterials, National Research Centre, Cairo, 11553, Egypt.
*Corresponding Author
Fatema Aziz Al-Sayed,
Department of Oral Biology, Faculty of Dentistry, Cairo University, Cairo, 11553, Egypt.
Tel: +201270333318
E-mail: fatema-aziz@dentistry.cu.edu.eg
Received: October 19, 2021; Accepted: November 10, 2021; Published: November 20, 2021
Citation: Fatema Aziz Al-Sayed, Dr. Radwa Hegazy, Zeinab Amin, Dr.Hanan El-Beherie. Combined Use of Hyaluronic Acid with Nano-bioactive Glass Enhanced Biocement Based Silicate Stimulated Bone Regenerative Capacity in Tibial Bone Defects of Rabbits: In-Vivo Study. Int J Dentistry Oral Sci. 2021;8(11):5033-5038. doi: dx.doi.org/10.19070/2377-8075-210001014
Copyright: Fatema Aziz Al-Sayed©2021. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: An ideal biomaterial for bone regeneration is a longstanding quest nowadays. Thisstudy aimed to evaluate
the osteogenic potentiality of nano-bioactive glass (NanoBG) enhanced biocement based silicate with or without hyaluronic
acid(HA) seeded in rabbits’ tibial bone defects.
Methodology: 24 male rabbits were divided into three equal groups. All rabbit's tibia had two defects 5mm in diameter (1
defect per tibia). Group 1(control): bone defects were left untreated. Group 2: defects received nanoBG enhanced biocement
based silicate cement. Group 3: defects received NanoBG cement mixed with HA. Animals in each group were divided equally
for euthanization after 3 weeks and after 6 weeks. At each duration, the bone specimens were processed and examined histologically
with histomorphometrically analysis of new bone area percentage.
Results: Thebone defects in group 3 showed significant improved osseous healing as compared to group 1&2 along the two
durations. Upon long duration healing, the histological examination of the bone defects of group 3 showed almost filled
defects with mature compact bone, however both groups 1&2 revealed less mature bone with more bone marrow spaces inbetween.
The morphometric analysis revealed a significant increase in the new bone area percentage in group 3 in comparison
to group 1 and 2 (P < 0.05).
Conclusions: The present study concluded that bone defects and fractures could be treated with NanoBG and HA cement.
Nano BG alone was capable of bone regeneration. Yet, the regenerative capacity of their combination was more significant.
2.Introduction
3.Materials and Methods
3.Results
4.Discussion
5.Conclusion
5.References
Keywords
Bone Regeneration; Bioactive Glass; BG; NanoBioactive Glass; Calcium Silicate Cement; Hyaluronic Aacid; Tibial Bone Defect.
Introduction
Bone defects represent a serious pathological condition that can
cause severe complications and affect vital components of the
bone. Bone fractures’ healing and union is an obstacle due to
precarious blood supply that maycomplicate the treatment [1, 2].
The demand for an ideal biosynthetic material for replacement
and repair of bone tissue loss has increased significantly due to
the complications of autografts, allografts and xenografts. Despite
the increasing number of these materials, there is no ideal
bone graft substitute [3, 4]. Bone tissue engineering (BTE) is an
advanced biomedical technique that is considered as an effective
approach for bone regeneration and reconstruction of lost
bone tissue. Currently, the paradigm of BTE depends on bone
substitute materials which can promote the human body’s own
regenerative capacity in the repair process by stimulating expression
of osteogenic genes. In this regard, the scaffold should be
designed as bone tissue “regeneration” rather than mere “replacement”[
5]. Synthetic materials used for bone regeneration include
metal materials, inorganic non-metallic materials, organic materials, and composites, have great potential in clinical applications.
Bioactive glass (BG) have been applied extensively for bone repair
and regeneration as they have shown excellent bone bioactivity
and in vivo-bone forming ability [6]. Nanoscale of BGshowed
improvements of its bioactivity, this can be explained by the
higher surface area of nanoscale BG thatpermits rapid release of
ions and a higher protein adsorption. Previous researches have
proven thatbone and teeth tissues mineralization were accelerated
when these tissues were in contact with nanoscale particles
in comparison with micron scaled particles [7]. Biocement based
silicate was developed more than 20 years ago. The main advantage
of silicate-based cements is the fact that Si plays an essential
role in mineralization and gene activation in bone regeneration
process. It was reported that silicate can be combined with Ca2+
ions, which have shown its superiority in pre-osseous and osseous
tissue repair in vitro and vivo [8, 9]. Calcium silicate cements
have been shown to facilitate cell attachment and integration with
opposing hard tissues as well as their capability in bone regeneration.
Many researchers reported that biomaterials containing
CaO–SiO2 enhances mineral deposition across their surfaces and
were found to bond to living bone and soft tissues through the
development of a biologic hydroxyapatite layer on the surface [4].
However, the degradation of pure tricalcium silicate cement is too
slow to match the rate of new bone formation, which limits its
application in bone regeneration [10]. Numerous studies reportedthe
efficacy of combining silicates with other materials in order
to design bioactive biomaterials with better properties for tissue
regeneration, especially bone tissue engineering applications [11].
Recently, hyaluronic acid (HA) act as an important natural polymer
that improves and modifies the biological properties of a
synthetic scaffold [12, 13]. HA was found to be capable of binding
to extracellular matrix molecules and cell surface receptors.
Subsequently, it helped in regulating cellular behaviour via control
of the tissues’ macro- and micro-environments [14]. It has been
proven that HA has a great role in angiogenesis, wound healing,
and tissue regeneration.HA-based scaffolds represented a source
for osteoinductive elements that can subsequently promote the
osteogenic effects of implanted scaffolds [12, 15]. Several previous
reports on the use of nano-bioactive glass, bioactive calcium
silicate cement and hyaluronic acid in bone regeneration were
found. Yet, none incorporated them together as a biocomposite
mixture. Therefore, this study aimed to introduce a novel composite
scaffold with extrudable nanostructured bioactive glass and
calcium silicate based biocement pastes using hyaluronic acid as a
solvent, which may provide surprising alternatives for bone tissue
regeneration.
Materials and Methods
Ethical Statement
The study protocol was approved from the Institutional Animal
Care and Use Committee (IACUC) - Cairo University. Approval
number (CU/III/F/46/19).
Experimental Animals
This experiment was conducted on 24 healthy male New Zealand
white rabbits weighing about 2.5 to 3.5 kg. Animals were
purchased and housed in the animal house Faculty of Medicine,
Cairo University. The rabbits were randomly allocated into three
groups. Each group consisted of 8 rabbits. Animals were kept in
separate cages and maintained under controlled temperature at
25°C± 2°C with 12 h light/dark cycle. They were fed pellets and
fresh tap water available ad libitum with good ventilation condition
throughout the experiment.
Bone defect preparation
The surgical procedure was performed under general anaesthesia
upon intramuscular injection of a combination of 5mg/kg Xyaline
2%(Xyla-Ject®, PhoenixTM, Pharmaceutical Inc.) and 40mg/
kg Ketamine Chlorhydrate ( Ketamine, Amoun pharmaceutical
company) [16]. A single bone defect 5 mm in diameter was created
in each tibia using a round surgical bur coupled to a low-speed
hand piece usedunder constant copious irrigation withphysiological
saline solution to prevent the overheating of the periphery of
the bone. The bone defects were drilled until the medullary canal
is reached. The defects of group 1 (control group) were left untreated
(filled with blood clot), while group 2 defects were filled
with nanoBG enhanced biocement based silicate mixed with distilled
water. Group 3 defects were packed with nanoBG enhanced
biocement based silicate mixed with HA. Postoperatively, the periosteum,
muscle and fascia were then repositioned properly over
the defects and sutured with resorbable #2.0 catgut and the skin
was sutured with interrupted #3.0 silk sutures. Systemic antibiotic
Amikacin® 1.5 mg/ kg (Amoun pharmaceutical company) was
administrated as an intramuscular injection per 12 hours for 1
week [17]. Analgesic 10 mg/kg cataflam (Novartis, Egypt) was
administrated to relieve postoperative pain and topical antibiotic
spray; Bivatracin (Egyptian Company for Advanced Pharma,
Egypt) to avoid local infection.Three weeks postoperatively,half
of the animals in each group were euthanized with an intra-peritoneal
overdose of Ketamine/Xylazine mixture,however, the
other half after 6 weeks(18).Both tibiae were dissected free from
any soft tissues; the bone specimens including the defect of each
group were cut by a disc under constant irrigation to include the
entire defect sites.
Histological and histomorphometry examination of H & Estained
sections
Bone specimens were fixed in 10% calcium formol solution for
48 hours and demineralized in 10% EDTA (El-Gomhouria co.)
solution for 4-5 weeks. The specimens were subsequently dehydrated
in ascending grades of alcohol, cleared in xylol, and then
embedded in paraffin blocks. Serial 5-6 µm paraffin cross sections
were cut with a microtome using diamond knife and mounted on
clean glass slides, and finally stained with H and E stain. Histomorphometric
analysis of the newly formed bone area percentage
was obtained using Leica Owen 500 image analyser Computer
system (Leica Imaging System Ltd., Cambridge, U.K. in Research
unit in faculty of Oral and Dental Medicine, Cairo University).
The image analyser consisted of a coloured video camera, coloured
monitor, hard disc of IBM personal computer connected
to the microscope and controlled by Leica Qwen 500 software.
Statistical analysis
The data obtained from the histomorphometric analysis were statistically
described in the terms of mean and standard deviation
(SD) values. ANOVA was used to compare different observation
times within the same group. Followed by Tukey’s post hoc test to compare multiple 2-group comparisons. The significance level
was set at p < 0.05. Statistical analysis was performed with IBM
SPSS 18.0 (Statistical Package for Scientific Studies, SPSS, Inc.,
Chicago, IL, USA) version 22 for windows.
Materials
Tetraethyl orthosilicate (TEOS), triethyl phosphate ethanol
(TEP), nitric acid (65%) used as a catalyst, calcium nitrate tetrahydrate
(Ca(NO3)2.4H2O), ammonia (NHOH), and silver nitrate
(AgNO3) were used to prepare the silver bioactive glass and calcium
silicate cement by sol-gel method. The silver bioactive glass
system formula reached was 60SiO2:35CaO:4P2O5:1 Ag2O3(19).
The novel biocement was prepared by mixing 80% of calcium
silicate cement to 20% of silver bioactive glass [20]. Either high
molecular weight hyaluronic acid (1750 kDa) (Sigma-Aldrich) or
distilled water was used to prepare the cement paste which was
subsequently moulded into the critical sized bone defect [21].
Results And Discussion
Transmission electron microscope (TEM) analysis of silver
nanoBGbased silicate biocement and silver nanoBGbased
silicate biocement /HA:
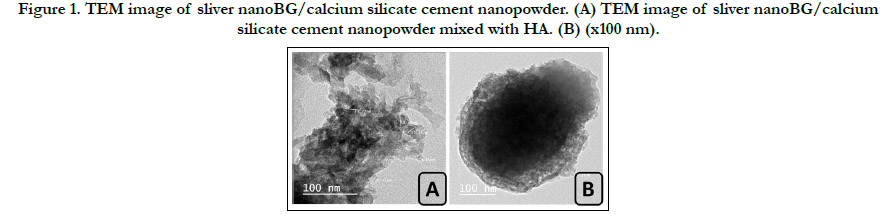
TEM analysis of novel sliver nanoBG/calcium silicate biocementshowed
heterogeneous shape of the nanoparticles with formation
of crystalline dark and amorphous transparent nanoparticles. The
average particle size of nanoparticles of the clumped distributions
was between 9.46 and 18.36 nm. (fig. 1A) While the TEM images
of novel biocement mixed with HA showed a uniform distribution
with large hydrated cloudy clusters encapsulating many nanoparticles
ofdifferent morphology. The average nanoparticles size
ranged from 12.09 to 15.31 nm in diameter. (fig. 1B).
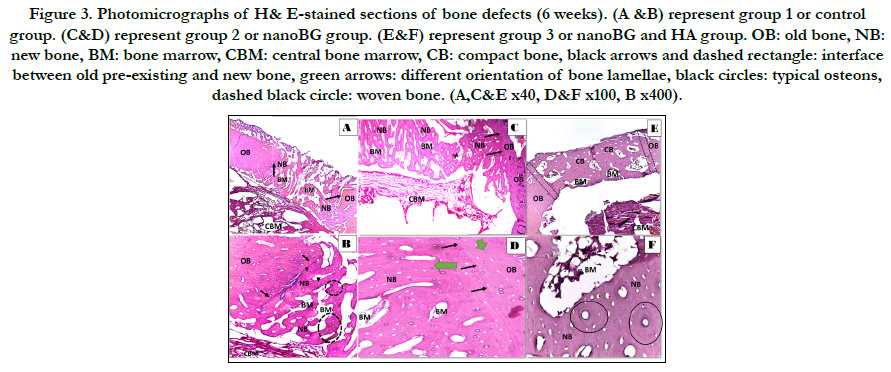
Histological (H & E stain) results
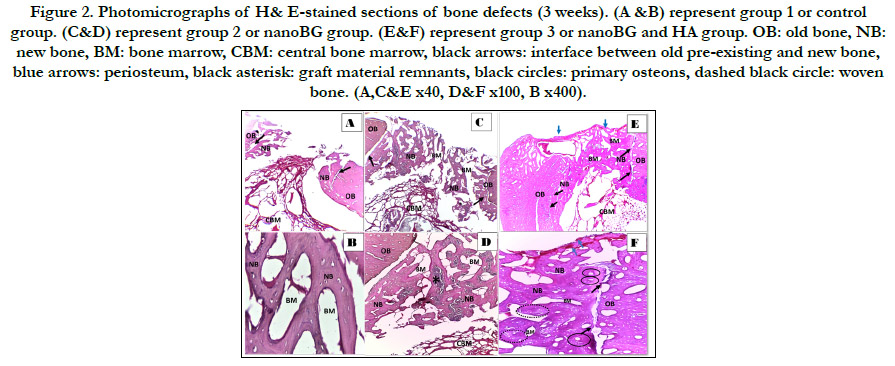
Three weeks postoperatively, group 1 showed almost open bone
defect with some granulation tissue in the middle of the defect
and few newly formed bone trabeculae at the edges enclosing
large bone marrow cavities in-between. (fig. 3 A&B) Thin and
interconnected neobone trabeculae were formed around the graft
material in group 2 with wide bone marrow cavities in-between.
(fig. 3 C&D) Group 3 revealed newly formed bone trabeculae
filling the defect with thick trabeculation and appearance of primary
osteons having wide haversian canals as well as scattered
areas of woven bone. Bone defect showed a highly vascularized
periosteum coverage. The interface between newly formed bone
and old pre-existing bone was about to be sealedwith scalloped
border. (fig. 3 E&F) Six weeks postoperatively, group 1 defects
revealed newly formed interconnecting bone trabeculae filling almost
all the defect as compared to the same group at 3 weeks
postoperatively. Dispersed areas of woven bone with different degrees
of basophilia were detected. (fig.4 A&B) Group 2 showed
bone defect almost filled with newly formed lamellar bone with
thick trabeculation enclosing smaller bone marrow spaces.Indistinguishable
interface was observed between old bone and newly
formed bone with significant difference in the orientation of the
lamellae between old and new. (fig.4 C&D) Group 3 demonstrated
completely restored defect with densely packed compact bone
tissue that could not be distinguished from the old bone with
completely sealed interface. Dense compact bone compromised
lamellae assumed in concentric arrangement around a haversian
canal, forming a typical osteon. (fig.4E&F).
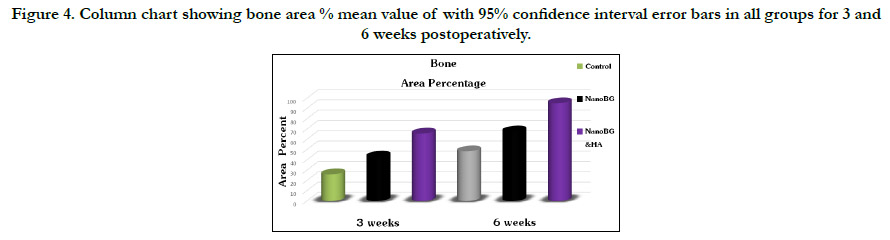
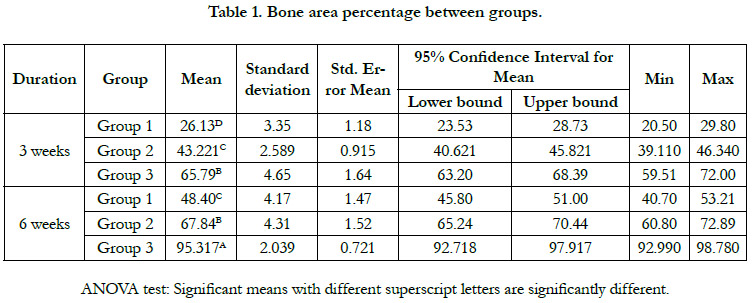
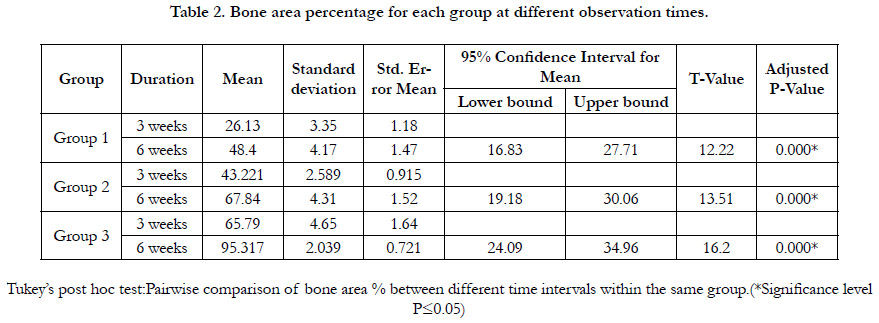
Histromorphometric and statistical analysis
The histomorphometric analysis of the bone area percentage between
groups during both time intervals showed the highest bone
area percent in group 3 which revealed a statistically significant
increase in the mean of bone area percent relative to group 1
and 2. Moreover, bone area percentage mean value significantly
increased by time in all groups.(fig. 4, table 1 and 2).
Discussion
Beforeevaluating biomaterials in human, a perfect bone substitute
ought to be tried in vitroand in vivo, to be certain beyond any
doubt that it works viably and securely. Therefore, establishingan
appropriate animal model is an essential step when assessing the
mechanical property and biocompatibility of bone tissue biomaterials
[22]. Silica based BG has been exclusively applied for bone
repair and regeneration as they showed excellent bone bioactivity
and in vivo bone forming ability. In this study, BG composite
was the material of choice with replacing the sodium component
with silver.Silver ions were found to be perfect in enhancing the
antibacterial and osteogenic activities [23]. Numerous literatures
indicates that HA acts primarily to promote healing at fracture site
by stimulating callus formation. Furthermore, HA of a specific
molecular weight when used in vitro, was reported to significantly
increase alkaline phosphatase activity and stimulate osteoblastic
cell proliferation and differentiation [24].
Through the current study nanoBG cement alone and upon addition
to HA promoted bone regeneration in critical-sized tibial
bone defects along short and long-time intervals however, histological and histomorphometric examinations revealed superior
results in HA groups at both time intervals.
The histological results in the nanoBG group at both time intervals
showed better bone regeneration than control group. They
showed interconnected bone trabeculae filling almost all the defect
perimeters which appeared thicker with smaller bone marrow
cavities 6 weeks postoperatively. Moreover, the bone area percentage
was significantly higher in nanoBG group. BG showed unique
properties in bone tissue regeneration by formation of carbonated
hydroxyapatite layer (HCA) when exposed to biological fluid.
This layer is responsible for the strong bonding between bioactive
glasses and human bone [11]. In coincidence with our findings,
Abirman et al., 2002; concluded that after 6 weeks of BG implantation
in tibial bone defects in rabbits the periosteal and the
endosteal regions were completely closed [25]. As well asPinto et
al., 2013; reported that tibial bone defects implanted with biosilicate
ceramics showed highly organized newly formed bone filling
the whole defect after 45 days postoperatively [26]. Another study
demonstrated that the quantitative woven bone volume was significantly
higher in BG group than in control group after 20 days
of implanting BG in tibial bone defects of rats [27].
NanoBG with HA group showed superior histological results
than the other 2 groups throughout the whole experiment. Newly
formed bone was observed filling the defect with thick trabeculation
and intimate bonding with the defect pre-existing old bone,
however this bone was more organized and uniform in form of
dense compact bone enclosing typical haversian systems after 6
weeks. Superior bone regenerative results seen in nanoBG and
HA group could be assumed to the characteristic role of HA
in cell adhesion, chemotaxis, differentiation, and proliferation,
signalled through several macromolecules and especially during
wound healing and tissue regeneration [28, 29]. Similarly, Shamma
et al., 2017; confirmed that addition of HA into bone graft
around dental implants placed in sockets of extracted mandibular
third premolar of dogs after 6 weeks showed entirely filled mature
well-formed bone with obvious complete osseointegration with
the native bone [30].
On contrary, Ahmed et al., 2020; revealed that HA implanted in
combination with biphasic calcium phosphate cement in femoral
bone defects of rats didn’t give superior bone regeneration
in comparison with the cement alone 4 and 10 weeks postoperatively.
They explained their findings by assuming that the low molecular
weight (less than 1000 kDa) of the HA used in their study
was the reason [31]. The HA ability to enhance the osteogenic and
osteoinductive properties of bone graft materials was dependent
on its dose and molecular weight. It was found that HA of higher
molecular weight (more than 1000 kDa) promoted mesenchymal
stem cells (MSCs) proliferation and differentiation [28]. This may
confirm the osseous regenerative potentiality of HA used in our
present study which had high molecular weight (1750 kDa).
Parallel to our results, Elkarargy, 2013; demonstrated that combining
HA to synthetic bone graft increased the new vital bone formation
bone area percentage upon implantation in sockets of extracted
lower lateral incisors in rabbits when compared with bone
graft alone and empty control group after 4weeks and 8weeks
postoperatively [32]. Moreover, Shirakata et al., 2021;concluded
that adding HA either alone or combined with collagen matrixin
5 mm intrabony defects on the walls of mandibular premolars in
dogs enhanced the periodontal wound regeneration [33].
Figure 1. TEM image of sliver nanoBG/calcium silicate cement nanopowder. (A) TEM image of sliver nanoBG/calcium silicate cement nanopowder mixed with HA. (B) (x100 nm).
Figure 2. Photomicrographs of H& E-stained sections of bone defects (3 weeks). (A &B) represent group 1 or control group. (C&D) represent group 2 or nanoBG group. (E&F) represent group 3 or nanoBG and HA group. OB: old bone, NB: new bone, BM: bone marrow, CBM: central bone marrow, black arrows: interface between old pre-existing and new bone, blue arrows: periosteum, black asterisk: graft material remnants, black circles: primary osteons, dashed black circle: woven bone. (A,C&E x40, D&F x100, B x400).
Figure 3. Photomicrographs of H& E-stained sections of bone defects (6 weeks). (A &B) represent group 1 or control group. (C&D) represent group 2 or nanoBG group. (E&F) represent group 3 or nanoBG and HA group. OB: old bone, NB: new bone, BM: bone marrow, CBM: central bone marrow, CB: compact bone, black arrows and dashed rectangle: interface between old pre-existing and new bone, green arrows: different orientation of bone lamellae, black circles: typical osteons, dashed black circle: woven bone. (A,C&E x40, D&F x100, B x400).
Figure 4. Column chart showing bone area % mean value of with 95% confidence interval error bars in all groups for 3 and 6 weeks postoperatively.
Acknowledgment And Declaration
The authors are very thankful to all the associated personnel in
any reference that contributed for the success of this research,
along with deep gratitude to Animal House of Faculty of Medicine
Cairo university staff and personnel for their care and endless
work dedication.
Funding statement: This research did not receive any specific
grant from funding agencies in the public, commercial, or notfor-
profit sectors.
Conclusion
From our study, we can conclude that the combined use of HA
and nanoBG enhanced silicate biocement for osteogenic regeneration
of osseous defects is a potential treatment alternative for
accelerated healing than using these biomaterials alone.This conclusion
is a new breakthrough in the field of bone graft materials
since BG overcomes the limitations associated with other synthetic
and natural bone grafts and make it promising bone substitute
material in critical bone defects in clinical applications.
References
-
[1]. Frencken JE, Sharma P, Stenhouse L, Green D, Laverty D, Dietrich T.
Global epidemiology of dental caries and severe periodontitis - a comprehensive
review. J ClinPeriodontol. 2017 Mar;44Suppl 18:S94-S105. PubMed
PMID: 28266116.
[2]. Chen M, Wright CD, Tokede O, Yansane A, Montasem A, Kalenderian E, Beaty TH, Feingold E, Shaffer JR, Crout RJ, Neiswanger K, Weyant RJ, Marazita ML, McNeil DW. Predictors of dental care utilization in northcentral Appalachia in the USA.Community Dent Oral Epidemiol. 2019 Aug;47(4):283-290. PubMed PMID: 30993747.
[3]. Tchicaya A, Lorentz N. Socioeconomic inequalities in the non-use of dental care in Europe. Int J Equity Health. 2014 Jan 29;13:7. doi: 10.1186/1475- 9276-13-7. PubMed PMID: 24476233.
[4]. Edelstein BL, Chinn CH. Update on disparities in oral health and access to dental care for America's children. AcadPediatr. 2009 Nov-Dec;9(6):415-9. PubMed PMID: 19945076.
[5]. Goettems ML, Ardenghi TM, Demarco FF, Romano AR, Torriani DD. Children's use of dental services: influence of maternal dental anxiety, attendance pattern, and perception of children's quality of life. Community Dent Oral Epidemiol. 2012 Oct;40(5):451-8. PubMed PMID: 22537392.
[6]. Yuen A, Rocha CM, Kruger E, Tennant M. The equity of access to primary dental care in Săo Paulo, Brazil: A geospatial analysis. Int Dent J. 2018 Jun;68(3):171-175. PubMed PMID: 28913887.
[7]. Piovesan C, Antunes JL, Guedes RS, Ardenghi TM. Influence of self-perceived oral health and socioeconomic predictors on the utilization of dental care services by schoolchildren. Braz Oral Res. 2011 Mar-Apr;25(2):143-9. PubMed PMID: 21359493.
[8]. M Orfali DS, S Aldossary DM. Utilization of Dental Services in Saudi Arabia: A Review of the Associated Factors. Saudi J Oral Dent Res 2020;05:147–9.
[9]. John JR, Mannan H, Nargundkar S, D'Souza M, Do LG, Arora A. Predictors of dental visits among primary school children in the rural Australian community of Lithgow. BMC Health Serv Res. 2017 Apr 11;17(1):264. PubMed PMID: 28399864.
[10]. AlHumaid J, El Tantawi M, AlAgl A, Kayal S, Al Suwaiyan Z, Al-Ansari A. Dental Visit Patterns and Oral Health Outcomes in Saudi Children. Saudi J Med Med Sci. 2018 May-Aug;6(2):89-94. PubmedPMID: 30787827.
[11]. El Bcheraoui C, Tuffaha M, Daoud F, Kravitz H, AlMazroa MA, Al Saeedi M, Memish ZA, Basulaiman M, Al Rabeeah AA, Mokdad AH. Use of dental clinics and oral hygiene practices in the Kingdom of Saudi Arabia, 2013.Int Dent J. 2016 Apr;66(2):99-104. PubMed PMID: 26749526.
[12]. Al Agili DE, Farsi NJ. Need for dental care drives utilisation of dental services among children in Saudi Arabia. Int Dent J. 2020 Jun;70(3):183-192. PubMed PMID: 31912900.
[13]. Al-Hussyeen AJ. Factors affecting utilization of dental health services and satisfaction among adolescent females in Riyadh City. Saudi Dent J. 2010 Jan;22(1):19-25. PubMed PMID: 23960475.
[14]. World Health Organization. Oral health surveys: basic methods. Fifth Edit. 2013.
[15]. Kassim S, Bakeer H, Alghazy S, Almaghraby Y, Sabbah W, Alsharif A. Socio- Demographic Variation, Perceived Oral Impairment and Oral Impact on Daily Performance among Children in Saudi Arabia. Int J Environ Res Public Health. 2019 Jul 10;16(14):2450. PubMed PMID: 31295837.
[16]. Alayadi H, Bernabé E, Sabbah W. Examining the relationship between oral health-promoting behavior and dental visits. Int J Health Sci (Qassim). 2019 May-Jun;13(3):40-43. PubMed PMID: 31123439.
[17]. Al Agili DE. A systematic review of population-based dental caries studies among children in Saudi Arabia. Saudi Dent J. 2013 Jan;25(1):3-11. Pub- Med PMID: 23960549.
[18]. Gaffar BO, Alagl AS, Al-Ansari AA. The prevalence, causes, and relativity of dental anxiety in adult patients to irregular dental visits. Saudi Med J. 2014 Jun;35(6):598-603. PubMed PMID: 24888660.
[19]. Eitner S, Wichmann M, Paulsen A, Holst S. Dental anxiety--an epidemiological study on its clinical correlation and effects on oral health. J Oral Rehabil. 2006 Aug;33(8):588-93. PubMed PMID: 16856956.
[20]. Badri P, Saltaji H, Flores-Mir C, Amin M. Factors affecting children's adherence to regular dental attendance: a systematic review. J Am Dent Assoc. 2014 Aug;145(8):817-28. PubMed PMID: 25082930.
[21]. Alshammary F, Aljohani FA, Alkhuwayr FS, Siddiqui AA. Measurement of Parents' Knowledge toward Oral Health of their Children: An Observational Study from Hail, Saudi Arabia. J Contemp Dent Pract. 2019 Jul 1;20(7):801-805. PubMed PMID: 31597799.
[22]. Alshahrani A, Raheel S. Health-care System and Accessibility of Dental Services in Kingdom of Saudi Arabia: An Update. J Int Oral Heal 2016;8:883– 7.
[23]. Al-Jaber A, Da'ar OB. Primary health care centers, extent of challenges and demand for oral health care in Riyadh, Saudi Arabia. BMC Health Serv Res. 2016 Nov 4;16(1):628. PubMed PMID: 27809919.
[24]. Reda SF, Reda SM, Thomson WM, Schwendicke F. Inequality in Utilization of Dental Services: A Systematic Review and Meta-analysis. Am J Public Health. 2018 Feb;108(2):e1-e7. PubMed PMID: 29267052.
[25]. Nishide A, Fujita M, Sato Y, Nagashima K, Takahashi S, Hata A. Income- Related Inequalities in Access to Dental Care Services in Japan. Int J Environ Res Public Health. 2017 May 12;14(5):524. PubMed PMID: 28498342.
[26]. Ramraj C, Sadeghi L, Lawrence HP, Dempster L, Quińonez C. Is accessing dental care becoming more difficult? Evidence from Canada's middle-income population.2013;8(2):e57377. PubMed PMID: 23437378; PMCID: PMC3577722.
[27]. Alsharif AT, Kruger E, Tennant M. Identifying and prioritising areas of child dental service need: a GIS-based approach. Community Dent Health. 2016 Mar;33(1):33-8. PubMed PMID: 27149771.