A Modified Free Gingival Graft Technique In Vertical And Horizontal Soft Tissue Augmentation Before Dental Implantation: A Randomized, Split-Mouth, Controlled Clinical Trial
Zayed Alnuaimi1, Majd Othman2, Imad Katbeh3*, Somia Naser4, Wilfrid Kamgang5, Olga Voeykova6, Mamasaidova Zarina7, SaidovaPatimat8
1 MSc Student, Faculty of Dentistry, Department of Periodontology, Damascus University, Syria.
2 Professor, Faculty of Dentistry, Department of Periodontology, Damascus University, Syria.
3 Assistant Professor, Department of Pediatric Dentistry and Orthodontics RUDN University (Peoples� Friendship University of Russia). Russia, Moscow.
4 MSc, Faculty of Dentistry, Department of Periodontology, Damascus University, Syria.
5 PhD student, Department of Pediatric Dentistry and Orthodontics. RUDN University, Russia.
6 PhD student, Department of Pediatric Dentistry and Orthodontics RUDN University, Russia.
7 Medical Institute, Peoples' Friendship University of Russia (RUDN University) Moscow, Russia.
8 Medical Institute.Peoples' Friendship University of Russia (RUDN University) Moscow, Russia.
*Corresponding Author
Imad Katbeh,
Assistant Professor, Department of pediatric dentistry and orthodontics, Peoples� Friendship University of Russia (RUDN University), 117198 Miklukho-Maklaya Street 6, Moscow,
Russia.
Tel: +79168268962
E-mail: katbeh@bk.ru
Received: October 01, 2021; Accepted: November 10, 2021; Published: November 15, 2021
Citation: Zayed Alnuaimi, Majd Othman, Imad Katbeh, Somia Naser, Wilfrid Kamgang, Olga Voeykova e al., A Modified Free Gingival Graft Technique In Vertical And Horizontal Soft Tissue Augmentation Before Dental Implantation: A Randomized, Split-Mouth, Controlled Clinical Trial. Int J Dentistry Oral Sci. 2021;8(11):5017-5022. doi: dx.doi.org/10.19070/2377-8075-210001011
Copyright: Imad Katbeh�2021. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
The presence of a sufficient amount of keratinized tissue around dental implants is directly related to their success, facilitates
the following restorative procedures, and obtains satisfactory cosmetic results.
This study aims to investigate the effectiveness and predictability of different treatment modalities to gain keratinized tissue
(KT) in partial edentulous jaws prior to dental implant placement; modified free gingival graft (MFGG), and connective tissue
graft (CTG).
Materials and Methods: A total of 14 (age range between 25 and 58 years) partially edentulous patients (28 sites) with insufficient
zones of keratinized tissue at the prospective implant positionswere included in this study, two treatment modalities
were performed in the lower jaw: MFGG, and CTG.
MFGG and CTG were applied in the premolar and molar positions randomly. Assessed outcomes up to 6 months postsurgery
included changes in width and thickness of keratinized tissue.
Results: After 6 months of follow-up,changes in keratinized tissue width demonstrated an increase of 2.29 � 1.6 mm in connective
tissue graft, whereas modified free gingival graft showed an increase of 3.95 � 1.25 mm, on the other hand changes
in keratinized tissue thickness demonstrated an increase of 0.85 � 0.5 mm in connective tissue graft, whereas modified free
gingival graft showed an increase of 0.71 � 0.4 mm.
Conclusion: The two methods were suitable to increase the width and thickness of KT over a 6-month period, although
CTG alone rendered roughly 34% less gain at KT width compared to MFGG.
2.Introduction
3.Materials and Methods
3.Results
4.Discussion
5.Conclusion
5.References
Keywords
Modified Free Gingival Graft; Augmentation; Keratinized Tissue; De-Epithelialization; Keratinized Gingiva; Connective Tissue Graft.
Introduction
The absence of an adequate amount of keratinized mucosa is
directly associated with inflammatory gingival manifestations,
bleeding on probing, and bacterial plaque aggregation, in addition
to poor cosmetic appearance [1-3].
Previous studies concluded that dental implants placed within
sites with a minimum amount of keratinized tissues(<2mm) are
more susceptible to inflammatory manifestations and the accumulation of bacterial plaque compared to dental implants placed
within sites with sufficient keratinized tissues (KT>2mm)[4].
Moreover, the presence of sufficient vertical thickness of soft tissue
around the implants prevents the occurrence of resorption
of the crestal bone, which can be witnessed with the presence of
thin mucous around the implants [5]. Therefore, careful management
of the soft tissues around the implants is an important and
essential factor for obtaining satisfactory aesthetic and functional
results in the long term [6].
Several previous studies have shown that the presence of a sufficient
amount of keratinized tissue around dental implants is directly
related to the success of the osseointegrated of dental implants,
which facilitates the following restorative procedures and
obtains satisfactory cosmetic results, and also allows maintaining
the health of the tissues around the implants [3, 7].
Many different and useful surgical techniques are used to improve
soft tissues, including pedicle flaps and soft tissue grafts [6].
From the beginning of the second half of the last century [1]
free gingival grafts were the main technique to increase both the
width of the keratinized tissue and the vestibular extension. That
indicates the reliability of this clinical procedure [2, 3, 7-10].
The aim of the present study was, therefore, to evaluate two
treatment modalities, modified free gingival graft (MFGG), and
connective tissue graft (CTG), to gain vertical and horizontal
soft-tissue growth in the posterior region of the mandible before
installation of a dental implant. In addition, to report outcomes 6
months following the surgical interventions.
Materials And Methods
Trial design
The present study was designed as a split-mouth randomized controlled
trial to evaluate two procedures for the gain of keratinized
tissue over a 6-month period after the surgical interventions.
The study protocol was approved by the scientific research and
postgraduate board ethics committee of Damascus University,
Damascus, Syria (protocol code 3516 and date of approval 8 July
2019). The patients and parents were blinded by not being provided
any information about the treatment group to which they
were allocated.
Study population and inclusion criteria
Patients meeting the following inclusion criteria were consecutively
recruited and enrolled at the Clinic of Periodontology, University
of Damascus, Syria, between 2019 and 2021:
� Signed informed consent.
� The patient (male or female) must be 18 years or older.
� The patient is able to comply with the study-related procedures
such as exercising good oral hygiene and attending all follow-up
procedures.
� The patient can fully understand the nature of the proposed
surgery and is able to provide a signed informed consent.
� Partial edentulous patients in need of implant therapy in the
mandible and ability to place dental implants in the premolar and
molar positions.
� A reduced width of keratinized tissue (< 2mm), measured from
the center of the two prospective mandibular implant positions to
the buccal mucogingival junction.
� A reduced thickness of keratinized tissue (< 2mm), measured at
the center of the two prospective mandibular implant positions.
� Well-fitted, new maxillary and mandibular prostheses.
� Generally healthy.
� Commitment to maintaining good oral hygiene.
� No systemic disease that could affect wound healing and prevent
implant placement.
Exclusion criteria for all subjects included the following
� Patient is a heavy smoker (> 10 cigarettes per day).
� Patient is an insulin-dependent diabetes.
� General contraindications for dental and/or surgical treatment
are present.
� The patient has a history of malignancy, radiotherapy, or chemotherapy
for malignancy within the past 5 years.
� The patient is pregnant or nursing.
� The patient is taking medications or having treatments, which
have an effect on mucosal healing in general (e.g. Steroids, large
doses of anti-inflammatory drugs).
� The patient has a disease, which affects connective tissue metabolism
(e.g., collagenases).
� The patient is allergic to collagen.
� Patients having participated in a clinical trial within the last 6
months.
Patients not meeting all inclusion criteria were excluded from the
study. Upon enrollment, alginate impressions of the mandible
were obtained and stents for measurements were designed and
fabricated at the laboratory.
The stent was designed in such a way that it allowed measuring
the thickness of keratinized tissue at the center of the prospective
implant positions and measuring the width of keratinized tissue
from the center of the prospective implant positions to the buccal
mucogingival junction.
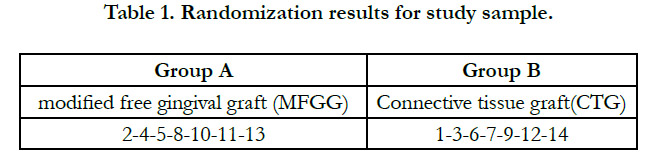
Randomization and Blinding
The clinically studied sample was randomly distributed using a
lottery, where the numbers from one to fourteen were written
on paper cards representing the research cases according to the
chronological sequence of their completion (No. 1 represents the
first case to be treated ... and so on) and then they were randomly
divided into two groups:
Group A (represents the group in which the right side was treated
with Modified free gingival graft).
Group B (represents the group in which the left side was treated
with the Connective tissue graft). The numbers shown in table (1)
carry the results of randomization of the study sample.
A double blinding was also adopted in this study so that both
the patient and the examiner were not know about the applied
method.
Surgical intervention
At the day of surgery, patients rinsed with 0.2% of chlorhexidine
solution and were given medication for pain relief (Mefenamic
acid, 500 mg). Subsequently, photographs of the two sites were
taken, the thickness of keratinized tissue was measured at the
center of the two prospective implant positions using a periodontal
probe and the prepared stent and the width of keratinized
tissue was measured on the buccal side of the two prospective
implant positions using a caliper and the prepared stent.
Following local anesthesia, two treatment modalities were then
randomly applied to the left and right side of the mandible:
In-group MFGG After an aseptic and antiseptic procedure, the
patient was anesthetized locally with articaine 4% 1:100.000
(Nova DFL, Rio de Janeiro, Brazil). A horizontal incision was
made under the bony crest of the edentulous space just below
the mucogingival line. Two vertical incisions of approximately 4
mm were made, one in the mesial and one in the distal area of
the edentulous space. The incision began in the mucogingival line
toward the bottom of the vestibular mucosa, with the aid of a
15C scalpel blade. The papilla was preserved. A full thickness flap
was made on the bony crest toward the lingual mucosa. Care was
taken to avoid perforation and to maintain a free space between
the mucosa and the bony crest. A partial thickness, 7 mm flap was
made on the vestibular mucosa.
The free gingival graft was removed from the palate region between
2.0 mm�3.0 mm thickness and 10.0 mm of length. Before
placement in the recipient bed, one part of graft was cascaded,
(epithelial tissue was removed) is the modification of the classical
technique of free gingival grafting [Figure 1]. The graft was positioned
to cover the entire surgical area. The bony crest portion of
the de-epithelialized graft was enveloped with the aid of a horizontal
mattress suture under the flap. Compression sutures were
made to stabilize the graft on the vestibular region of mandible.
In-group CTG the same bed preparation procedures were performed
in MFGG group except for preserving the mucosa resulting
from lifting the partially thickened vestibular flap to cover the
connective tissue graft.
The connective tissue graft was removed from the region in similar
measurement of MFGG and placed in the recipient bed in a
manner similar to the MFGG in addition to covering the CTG
with vestibular mucous.
Patients were given medications for pain relief (Mefenamic acid,
500 mg every 8 h), as well as a disinfectant solution (chlorhexidine
digluconate, 0.2% solution for rinsing every 8 h for a period of
7 days). No adaptations were made to the mandibular prostheses
and patients were not allowed to wear them during the entire
study period. On day 7 post-surgery, all patients were recalled for
suture removal, clinical measurements, and clinical photographs.
Outcome measures
The width (WKM) and thickness (TKM) of the keratinized mucosa
were the clinical parameters assessed for the included subjects.
WKM was measured on the buccal aspect tissue from the
center of the prospective implant positions to the mucogingival
junction positions using a caliper and the prepared stent. The
TKM was measured at the center of the prospective implant positions
using a UNC-15 probe (Hu-Friedy, USA). The parameters
were assessed prior to surgical procedure (baseline), 4 weeks, 12
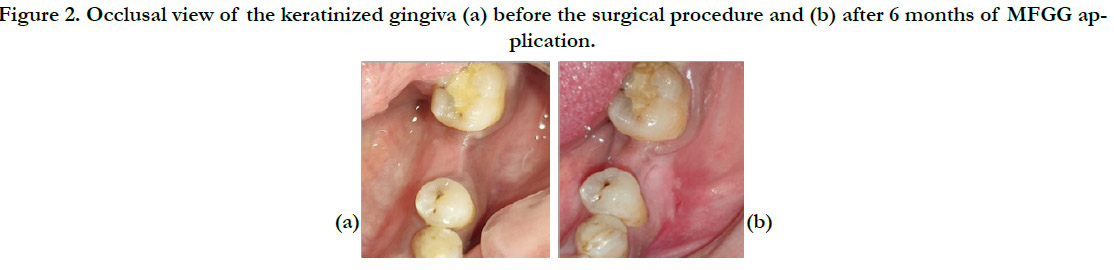
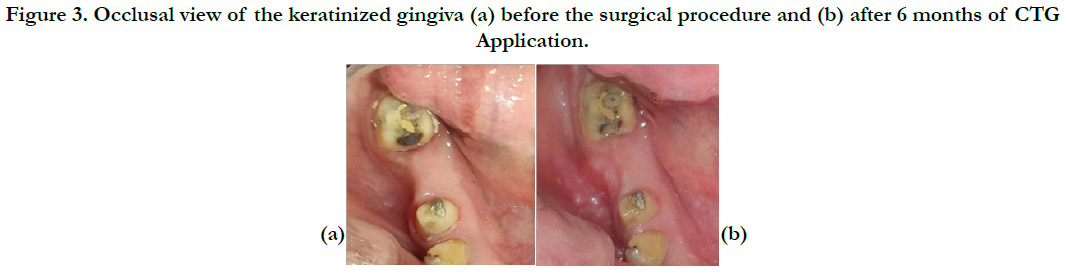
weeks and 6 months after surgery [Figure2, Figure3].
Statistical analysis
The recorded data was tabulated in Microsoft Excel (MS office
version 2010). Data analysis was done using the Windows
PC based software �MedCalc Statistical Software� version 13.3.1
(MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.
org; 2014). All testing was done at alpha 0.05 (95% confidence
limits). Intra- and inter-group comparison was performed using
the Wilcoxcon and Mann-Whitney U, respectively. Differences
above the 95% confidence intervals were regarded as statistically
significant.
Results
Fourteen partially edentulous patients (28 sites) with an age range
between 25 and 58 years (mean 41.5 years) were included in the
study and underwent soft-tissue augmentation surgeries. All patients received two treatment modalities, healing was generally uneventful,
and no local infection was observed at suture removal.
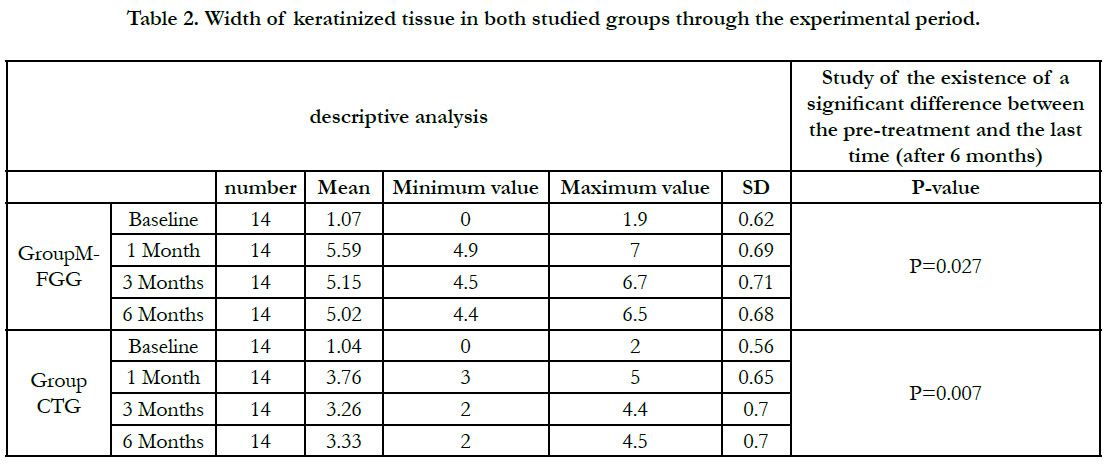
Width of keratinized tissue
At baseline, there was minimal WKM noted (group A, 1.07mm
� 0.62mm; group B, 1.04mm � 0.56mm) (p = 0.76). At 4 weeks,
12 weeks, and 6 months after surgery, the WKM significantly
increased for both the groups from baseline. The WKM at 4
weeks,12 weeks and 6 months for group A(5.59mm � 0.69mm,
5.15mm � 0.71mm, 5.02mm � 0.68mm) was significantly higher
than group B(3.76mm � 0.65mm, 3.26mm � 0.70mm, 3.33mm �
70mm) (p < 0.001) Table (2).
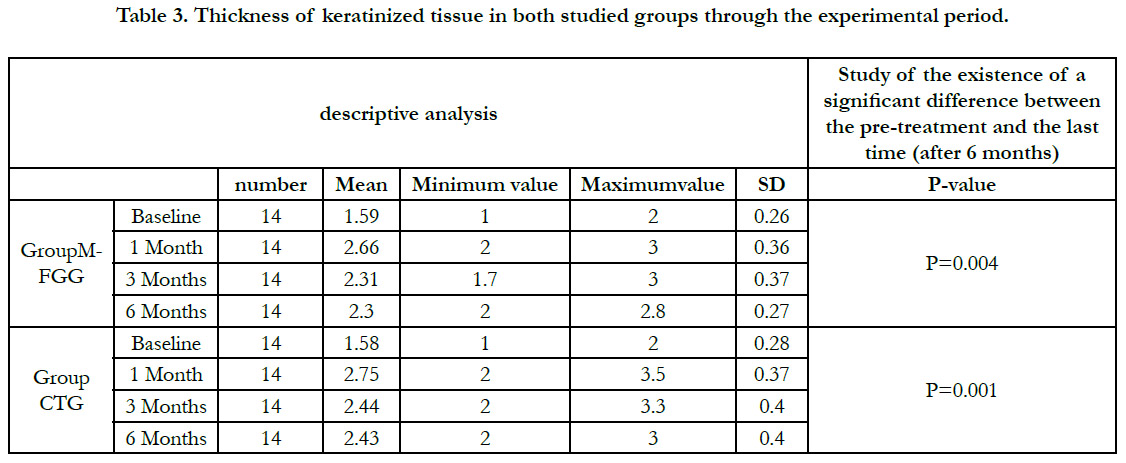
Thickness of keratinized tissue
The mean TKM at baseline was 1.59mm � 0.26mm for group A
and 1.58mm � 0.28mm for group B (p =0.94).
However, at 4 weeks, 12 weeks and 6 months, there was no statistically
significant difference in TKM between group B (2.75mm �
0. 37 mm, 2.44 mm � 0.40 mm, 2.43 mm � 0.40 mm) and group
A (2.66mm � 0.36 mm, 2.31 mm � 0.37 mm, 2.30 mm � 0.27
mm) (p = 0.61) (p = 0.49) (p = 0.44) respectively. Table (3).
Figure 2. Occlusal view of the keratinized gingiva (a) before the surgical procedure and (b) after 6 months of MFGG application.
Figure 3. Occlusal view of the keratinized gingiva (a) before the surgical procedure and (b) after 6 months of CTG Application.
Table 1. Mean pain scores for the 2 groups according to the 0�4 Scale (Kruskal�Wallis nonparametric test).
Table 2. Mean pain scores for the 2 groups according to the 0�4 Scale (Kruskal�Wallis nonparametric test).
Table 3. Mean pain scores for the 2 groups according to the 0�4 Scale (Kruskal�Wallis nonparametric test).
Discussion
Procedures to increase the soft tissue dimensions around the implants
can be applied during different stages of the dental implant
process such as before implantation, during the bone grafting
procedure, in conjunction with the implantation process, during
the bone osseointegration stage, at the temporization phase,
before the delivery of the final prosthesis or during the second
surgical stage [11].
Free gingival graft has been used for many years, as a successful
and predictable technique to increase the dimensions of the gingival
keratinized tissues, which prevents the occurrence of problems
at the level of soft and hard tissues after the rehabilitation of
the mouth with dental implants [8-10].
However, this technique requires two surgical sites, which may
cause pain, discomfort, and a sense of dissatisfaction by the patient,
in addition to the difference in color and texture from the
surrounding soft tissues and some shrinkage of the gingival graft,
all that leads to unsatisfactory aesthetic results [8-10].
In this study, a modification was made to the free gingival graft
technique to obtain soft tissue gain in both vertical and horizontal
directions in the posterior region of the lower jaw before dental
implantation.
With this modification, part of the gingival graft is de-epithelialized
and enters the submucosa at the top of the bone crest to
obtain a tissue gain in the vertical direction, and the other part is
fixed on the vestibular flap to increase the width of the keratinized
mucosa. This technique is superior to the traditional technique
in that one part of the graft is placed on top of the bone
crest, allowing a soft tissue gain in both the vertical and horizontal
directions [12].
The presence of adequate width of the keratinized mucosa is important
for the preservation of the gingival tissue, as many studies
have reported that the thick keratinized mucosa provides better
oral hygiene, which reduces the accumulation of plaque, bleeding,
inflammation, and gingival recession [2, 4].
The presence of thickened mucosa in both the vertical and horizontal
directions is very important in the process of dental implants.
Where the thick mucosa helps prevent early resorption
of the crest bone and facilitate the process of placing dental implants,
as it provides an increase in the stability of the area around
the implants and allows the patient to maintain appropriate oral
hygiene [1, 8, 9].
The results of this study showed a significant improvement in the
soft tissue thickness at the implant site, without a significant difference
between the two study groups.
Both study groups recorded a statistically significant change in
the soft tissue thickness index during the different measurement
intervals.
These results are consistent with studies that recorded an increase
in soft tissue volume after the use of a connective tissue graft at
a rate ranging between 0.35-3.2 mm, according to the site and the
follow-up periods [13-15].
In both study groups, we noticed a slight change in tissue thickness
between the first follow-up after a month of surgery and
the last follow-up after 6 months, and this is because most of the
changes occur in the gingival graft during the month of vaccination,
and that is in agreement with Thoma D.S. et al. study [16].
While the results of soft tissue thickness assessment between the
two groups showed no statistically significant difference after six
months (p=0.440) of surgery. The results of this study showed
a significant improvement in the width of the attached gingiva
at the implantation site, with a significant difference between the
two groups.
Both study groups recorded a statistically significant change in
the indicator of the width of the attached gingiva in different
follow-up periods.
Studies have found an increase in the width of the keratinized
gingiva after using a connective tissue graft placed under the slide
in all forms of mucosal gingival surgery techniques [17-19].
As for the increase in the width of the keratinized tissue around
the teeth or dental implants using a free gingival graft, it is documented
in many studies [20-24].
Our results are in agreement with Thoma et al study [14] that
compared different methods of tissue improvement around implants.
We also observed a change in the soft tissue width in the study
groups between the first follow-up after one month of surgery
and the last follow-up after 6 months, where the soft tissue width
decreased in the modified free graft group after 6 months by 0.57
mm than after a month of follow-up and in the connective graft
group, the width of the graft decreased after 6 months by 0.43
mm. This is due to the shrinkage of the grafts that occurred during
their maturation, full recovery (shrinkage), as the phenomenon
of graft shrinkage occurs during the healing process despite
being placed on an exposed bone, fixing it well and covering the
connective grafts with full-thickness slices [12, 17, 18]. The thickness
was similar in both groups andshowed no statistically significant
difference after six months (p=0.440) of surgery.
Conclusion
Within the limitations of the study, it was noted that the modified
free gingival graft technique increased the width of the keratinized
mucosa before dental implantation over the observation period of 6 months, on the other handconnective tissue graft alone rendered
roughly 34% less gain at keratinized tissue width compared
to modified free gingival graft. At the same time, it increased the
thickness of keratinized mucosa asmuch as the subepithelial connective
tissue graftdid.
References
-
[1]. Bjorn H. Free transplantation of gingival propria. Odontol Revy. 1963; 14:
323.
[2]. Narayan SJ, Singh PK, Mohammed S, Patel RK. Enhancing the zone of keratinized tissue around implants. J Indian Prosthodont Soc. 2015 Apr-Jun; 15(2):183-6. PMID: 26929509.
[3]. Souza AB, Tormena M, Matarazzo F, Ara�jo MG. The influence of periimplant keratinized mucosa on brushing discomfort and peri-implant tissue health. Clin Oral Implants Res. 2016 Jun; 27(6): 650-5. PMID: 26474541.
[4]. Gobbato L, Avila-Ortiz G, Sohrabi K, Wang CW, Karimbux N. The effect of keratinized mucosa width on peri-implant health: a systematic review. Int J Oral Maxillofac Implants. 2013 Nov-Dec; 28(6): 1536-45. PMID: 24278922.
[5]. Linkevicius T, Apse P, Grybauskas S, Puisys A. Influence of thin mucosal tissues on crestal bone stability around implants with platform switching: a 1-year pilot study. J Oral Maxillofac Surg. 2010 Sep; 68(9): 2272-7. PMID: 20605308.
[6]. Cairo F, Pagliaro U, Nieri M. Soft tissue management at implant sites. J Clin Periodontol. 2008 Sep; 35(8 Suppl):163-7. PMID: 18724848.
[7]. Bouri A, Bissada N, Al-Zahrani MS, Faddoul F, Nouneh I. Width of keratinized gingiva and the health status of the supporting tissues around dental implants. Int J Oral Maxillofac Implants. 2008 Mar-Apr; 23(2): 323-6. PMID: 18548930.
[8]. Oh SL, Masri RM, Williams DA, Ji C, Romberg E. Free gingival grafts for implants exhibiting lack of keratinized mucosa: a prospective controlled randomized clinical study. J Clin Periodontol. 2017 Feb; 44(2): 195-203. PMID: 27930813.
[9]. Marin DO, Leite AR, N�coli LG, Marcantonio C, Compagnoni MA, Marcantonio E. Free Gingival Graft to Increase Keratinized Mucosa after Placing of Mandibular Fixed Implant-Supported Prosthesis. Case Rep Dent. 2017; 2017: 5796768. PMID: 28293441.
[10]. Agarwal C, Tarun Kumar AB, Mehta DS. Comparative evaluation of free gingival graft and AlloDerm(�) in enhancing the width of attached gingival: A clinical study. Contemp Clin Dent. 2015 Oct-Dec; 6(4): 483-8. PMID: 26681852.
[11]. Thoma DS, Benic GI, Zwahlen M, H�mmerle CH, Jung RE. A systematic review assessing soft tissue augmentation techniques. Clin Oral Implants Res. 2009 Sep; 20 Suppl 4: 146-65. PMID: 19663961.
[12]. Imano MH, Cunha EJ, Storrer CLM, Deliberador TM. A modified free gingival graft technique for gaining vertical and horizontal soft tissue augmentation. J Indian Soc Periodontol. 2019 Jan-Feb; 23(1): 77-80. PMID: 30692749.
[13]. Eghbali A, De Bruyn H, Cosyn J, Kerckaert I, Van Hoof T. Ultrasonic Assessment of Mucosal Thickness around Implants: Validity, Reproducibility, and Stability of Connective Tissue Grafts at the Buccal Aspect. Clin Implant Dent Relat Res. 2016 Feb; 18(1): 51-61. PMID: 25040828.
[14]. Thoma DS, Buranawat B, H�mmerle CH, Held U, Jung RE. Efficacy of soft tissue augmentation around dental implants and in partially edentulous areas: a systematic review. J Clin Periodontol. 2014 Apr; 41 Suppl 15: S77- 91. PMID: 24641003.
[15]. Simion M, Rocchietta I, Fontana F, Dellavia C. Evaluation of a resorbable collagen matrix infused with rhPDGF-BB in peri-implant soft tissue augmentation: a preliminary report with 3.5 years of observation. International Journal of Periodontics and Restorative Dentistry. 2012 Jun 1; 32(3): 273.
[16]. Thoma DS, Zeltner M, Hilbe M, H�mmerle CH, H�sler J, Jung RE. Randomized controlled clinical study evaluating effectiveness and safety of a volume- stable collagen matrix compared to autogenous connective tissue grafts for soft tissue augmentation at implant sites. J Clin Periodontol. 2016 Oct; 43(10): 874-85. PMID: 27310522.
[17]. Schmitt CM, Br�ckbauer P, Schlegel KA, Buchbender M, Adler W, Matta RE. Volumetric soft tissue alterations in the early healing phase after peri-implant soft tissue contour augmentation with a porcine collagen matrix versus the autologous connective tissue graft: A controlled clinical trial. Journal of Clinical Periodontology. 2021 Jan; 48(1): 146-63.
[18]. Aroca S, Moln�r B, Windisch P, Gera I, Salvi GE, Nikolidakis D, et al. Treatment of multiple adjacent Miller class I and II gingival recessions with a Modified Coronally Advanced Tunnel (MCAT) technique and a collagen matrix or palatal connective tissue graft: a randomized, controlled clinical trial. Journal of clinical periodontology. 2013 Jul; 40(7): 713-20.
[19]. T�z�m TF, Dini FM. Treatment of adjacent gingival recessions with subepithelial connective tissue grafts and the modified tunnel technique. Quintessence Int. 2003 Jan; 34(1): 7-13. PMID: 12674352.
[20]. Raoofi S, Asadinejad SM, Khorshidi H. Evaluation of Color and Width of Attached Gingiva Gain in Two Surgical Techniques: Free Gingival Graft and Connective Tissue Graft Covered By Thin Mucosal Flap, a Clinical Trial. J Dent (Shiraz). 2019 Dec; 20(4): 224-231. PMID: 31875168.
[21]. Happe A, Stimmelmayr M, Schlee M, Rothamel D. Surgical management of peri-implant soft tissue color mismatch caused by shine-through effects of restorative materials: one-year follow-up. Int J Periodontics Restorative Dent. 2013 Jan-Feb; 33(1): 81-8. PMID: 23342350.
[22]. Moses O, Artzi Z, Sculean A, Tal H, Kozlovsky A, Romanos GE, et al. Comparative study of two root coverage procedures: a 24-month follow-up multicenter study. J Periodontol. 2006 Feb; 77(2): 195-202. PMID: 16460244.
[23]. Nemcovsky CE, Artzi Z, Tal H, Kozlovsky A, Moses O. A multicenter comparative study of two root coverage procedures: coronally advanced flap with addition of enamel matrix proteins and subpedicle connective tissue graft. J Periodontol. 2004 Apr; 75(4): 600-7. PMID: 15152826.
[24]. da Silva RC, Joly JC, de Lima AF, Tatakis DN. Root coverage using the coronally positioned flap with or without a subepithelial connective tissue graft. J Periodontol. 2004 Mar; 75(3): 413-9. PMID: 15088880.