Tumor Thickness and Cervical Nodal Metastasis in N0 Oral Tongue Squamous Cell Carcinoma Patients: A Prospective Study
Samer issa1, Omar Heshmeh2, Issam Alameen3, Zuhair Al-Nerabieah4*
1 PhD Candidate, Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Damascus University.
2 Professor, Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Damascus University.
3 Professor, Department of Otolaryngology, Faculty of Medicine -Al Mouwasat University Hospital- Damascus University.
4 PhD Candidate, Department of Pediatric Dentistry, Faculty of Dentistry, Damascus University, Damascus, Syria
*Corresponding Author
Zuhair Al-Nerabieah,
PhD Candidate, Department of Pediatric Dentistry, Faculty of Dentistry, Damascus University, Damascus, Syria.
E-mail: Zuhairmajid@gmail.com
Received: October 05, 2021; Accepted: October 28, 2021; Published: November 03, 2021
Citation: Samer Issa, Omar Heshmeh, Issam Alameen, Zuhair Al-Nerabieah. Tumor Thickness and Cervical Nodal Metastasis in N0 Oral Tongue Squamous Cell Carcinoma Patients: A Prospective Study. Int J Dentistry Oral Sci. 2021;8(10):4897-4901. doi: dx.doi.org/10.19070/2377-8075-21000990
Copyright: Zuhair Al-Nerabieah©2021. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Tumor Thickness (TT) plays an important role in the progress and prognosis of malignant tumors in general
and oral squamous cell carcinoma in particular. Many studies have concluded that thicker tumors were associated with higher
incidence of regional lymph node metastasis and as a result were associated with more lower survival rates.
Aim of Study: This study aimed to evaluate relation between tumor thickness (TT) and regional lymph node metastasis in
oral tongue squamous cell carcinoma patients, and to evaluate (TT) as a prognostic factor for lymph node metastasis and as
an influencer in the suggested treatment plan.
Materials and Methods: The study sample contained 40 patients (23 male, 17 female), who were diagnosed with stage I/II
oral tongue squamous cell carcinoma. A surgical procedure for tumor excision and an excisional biopsy was performed. The
tumor thickness was measured by one pathologist and the regional lymph nodes status was evaluated pathologically or radiologically
or by the two methods. The study sample was divided into three groups according to tumor thickness: TT<3mm, TT
(3-6mm), and TT>6mm, and the incidence of regional node metastasis in the three studied thickness groups was calculated.
Tumor thickness values were compared in cases of positive regional lymph node involvement and negative regional node
involvement using t-test.
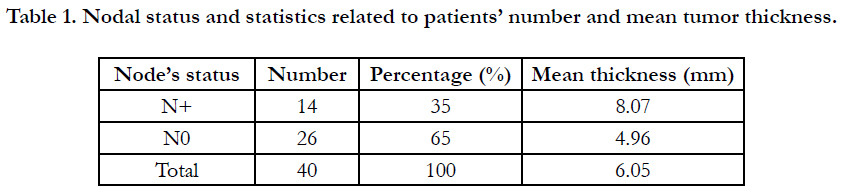
Results: Regional lymph node metastasis had occurred in 14 patients of our whole sample (35%) and the rates of nodal involvement
in the groups of thickness <3mm, 3-6mm, and >6mm were 18.18%, 33.33%, and 47.05% respectively. The mean
tumor thickness was 8.07mm in the positive lymph nodes group and 4.96 mm in the negative lymph node group with statistically
significant difference at p-value<0.05.
Conclusion: There was a higher incidence of regional lymph node metastasis in patients with thicker oral tongue SCC tumors,
also there was a critical high incidence of nodal involvement in OTSCC tumors that exceeded 3mm thick. Prophylactic neck
dissection or irradiation and close monitoring should be considered for those patients with more than 3mm thick tongue
tumors.
2.Introduction
3.Materials and Methods
3.Results
4.Discussion
5.Conclusion
5.References
Keywords
Oral Tongue Cancer; Lymph Node Metastasis; Tumor Thickness.
Introduction
The majority of oral cavity cancers affects the lower parts of
mouth and especially tongue margins and the neighboring floor
of mouth extending backwards to oropharynx. Oral tongue squamous
cell carcinoma (OTSCC) is one of the most common types
of oral cancers. It represents 25- 50% of oral cavity cancers and
includes only the lesions of the anterior tow thirds of tongue. [1,
2].
Tongue SCC (TSCC) is considered one of the most aggressive
types of OSCC. It tends to develop regional lymph node metastasis
earlier and with more incidence compared with the most
other kinds of OSCC,[3-6] also it has in its early stages the larger
mortality rates among various kinds of OSCC. [7]
TNM classification system for tumors staging has been founded
on for many decades. It has formed the reference for clinicians
and pathologists in determining the disease stage and prognosis
and has provided the standard guidelines for tumors treatment.
[8] Clinical practice has demonstrated some deficiency in this classification.
There were many failure cases that were encountered
when the suggested TNM treatment plan was applied, in addition
to that there were some N0 cases of OSCC which did not
respond -as expected- to the standard treatment plan determined
according to this classification. This opinion was supported by
many clinical studies which have confirmed the TNM classification
deficiency in predicting cervical node metastasis, disease recurrence
and survival. [9-15] and have concluded the presence
of many other prognostic factors - other than tumor size - that
influence the disease progression and prognosis such as tumor
thickness (TT) or depth of invasion (DOI), tumor differentiation
grade, perineural invasion, and many other factors.[16-20]
Many recent studies have confirmed on the role of tumor thickness
or depth of invasion in predicting OSCC regional lymph
node metastasis [21-24]. According to these studies the increase
in tumor thickness is associated with higher incidence of regional
lymph node metastasis. The minute knowledge of the role of tumor
thickness in predicting nodal metastasis in TSCC patients
could be resulted in much more control of the disease and as a
result much more survival for those patients by revising the TNM
suggested treatment plan which could be included in some cases
more additional prophylactic procedures and a modified followup
regime, so that in cases of large thickness TSCC tumors the
treatment plan may include an elective neck dissection or irradiation
or at least more close follow-up procedures.[10, 25]
The relation between tumor thickness and nodal metastasis in
OSCC patients has been explored in many studies. [21, 23, 24]
Concerning tongue SCC, most of this studies have evaluated
tongue SCC as a part of a sample population based on OSCC
patients, and few studies evaluated tongue SCC in particular, so
that the relation between tumor thickness and nodal involvement
in TSCC patients still need more studying as every subtype of
OSCC has its specific characteristics related to tumor nature,
clinical behavior, and disease development and prognosis. This
aforementioned reason makes the studying of each subtype of
OSCC separately an issue of great importance.[20, 25]
Materials And Methods
A prospective study was carried out on patients with initial diagnosis
of stage I/II oral tongue SCC who were accepted for
treatment at Al Moasat Hospital - Damascus University – in the
period between 2016 and 2020. Initially all patients suspected to
have Oral Tongue SCC had the routine clinical and radiological
examination for tumor diagnosis and staging.
There were forty patients with OTSCC who had met our inclusion
criteria and were included in our prospective study sample.
All patients included in the study underwent a radiological examination
consisted of plain radiographs, sonography, CT scan and
-when needed- MRI.
All patients underwent a surgical procedure for tumor excision
(partial glossectomy) and an excisional biopsy was obtained and
studied by the same pathology lab. The histological grade and the
maximum tumor thickness were determined by the pathologist
depending on the followed standard histological criteria.
Initially we excluded all patients suffering from recurrent or previously
treated tumors or suffering from tumor metastasis that had
affected the regional nodes or extended beyond it such as patients
with distant organs metastasis or patients with end-stage tumors.
Patients with predictive factors –other than tumor thickness- that
known to influence the incidence of regional lymph nodes metastasis
were also excluded of our sample such as patients with T3,
T4 tumors, patients with histological grade III tumors, and patients
with pathologically confirmed perineural or angiolymphatic
vessels invasion because of the high probability of these types of
tumors to develop regional lymph node metastasis.
On the other hand, all patients with exophytic type tumors and all
cases that had been diagnosed as carcinoma in situ were also excluded
because of the low probability of these tumors to develop
regional lymph node metastasis.
The study sample was divided into two groups depending on the
presence of regional lymph node metastasis:
1-Patients with positive lymph node metastasis (N+).
2-Patients with negative lymph node metastasis (N-).
Also, the study sample was divided into three groups according to
the maximum tumor thickness:
1-Group 1: Patients with tumor thickness <3 mm.
2-Group 2: Patients with tumor thickness 3-6 mm.
3-Group 3: Patients with tumor thickness >6 mm.
Regional lymph nodes status was determined depending on either
neck dissection and consequent pathological study performed on
the surgically obtained nodes specimen or on consequent radiological
and pathological findings obtained during the follow-up
period. The regional lymph nodes were considered positive (N+)
if the pathological result of neck dissection was positive or if
there was a new radiologically or pathologically confirmed lymph
node metastasis that had occurred during the follow- up period.
All patients included in our study were followed–up clinically and
radiologically and when needed histologically for a minimum period
of 16 months. During the follow-up period all patients suffered
from new regional lymph node metastasis were classified in
the positive nodal group (N+), on the other hand all patients who
did not suffer from new regional lymph node metastasis were
classified in the negative nodal group (N-).
When the clinical and radiological and histological examinations
were accomplished the TNM staging was established and the
standard therapy was offered for all study patients which contained
the surgical therapy alone or accompanied by one or more
one kind of treatments (radio or chemo therapy).
Statistical analysis was performed using computed program SPSS
Statistics software (Ver 23: IBM, Armonk, NY, USA). T-test was
used to analyze the difference in tumor thickness values between
the two studied nodal groups, with all statistics were considered
significant at p value<0.05.
Results
40 patients with stage I/II OTSCC were included in our study (23
male, 17 female) with a mean age 65.3 years (range: 42 - 80 years).
All patients underwent a surgical procedure for tumor excision
with or without neck dissection and were followed-up for a mean
period of 28 months (range:16.- 38 months).
The surgical procedure consisted of partial glossectomy, and the
decision of associated neck dissection was taken depending on
patient's clinical and histological findings. Neck dissection was
performed if there was an evident risk of subclinical nodal involvement
such as some large T2 tumors or rapidly growing tumors,
on the other hand when there was no evident risk of nodal
metastasis neck dissection was not performed and nodes status
was determined depending on adjunctive findings obtained during
the follow-up procedures.
According to the size of tumor there were 21 patients with T1
tumor (52.5%), and 19 patients with T2 tumor (48.5%). According
to the histological grade there were 27 cases of grade I tumors
(71%) and 13 cases of grade II tumors (29%).
Regional lymph node metastasis was discovered in 14 patients of
our complete sample (35%), 9 cases (22.5 %) of them were discovered
histologically after elective neck dissection, and 5 cases
(12.5%) were discovered later during the follow-up period.
Tumor Thickness in our whole sample ranged between 1 and 14
mm (mean: 6.05 mm), with a range of (2- 14 mm) in the positive
lymph nodal group (mean: 8.07 mm), and a range of (1 - 11 mm)
in the negative lymph nodal group (mean: 4.96 mm).
The positive lymph nodal group was characterized by higher values
of tumor thickness compared with the negative nodal group
and the difference in TT values was statistically significant (t-test,
p value= 0.006). These results demonstrated that increased tumor
thickness values were associated with more incidence of nodal
involvement in our OTSCC sample.
Table (1) Nodal status and patients' statistics are illustrated in table
1.
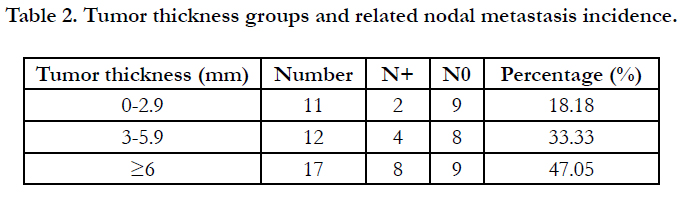
Eleven patients were included in Group 1 of thickness (<3mm),
twelve patients in group 2 (3-6mm), and seventeen patients
in group 3 (>6mm). Two cases of the 11 patients included in
group 1 had regional node metastasis (18.18%), 4 cases in group 2
(33.33%), and 8 cases in group 3 (47.05%). There was a progressive
increase in the incidence of regional nodal metastasis from
the thinner tumors’ groups to the thicker tumors’ groups which
was an expected result because of the aforementioned statistical
relation between regional nodal involvement and the increased
tumor thickness values.
Table (2) Tumor thickness groups and the related nodal metastasis
incidence are illustrated in table 2.
Discussion
TNM tumor classification system depends on tumor size in determining
disease stage and expected prognosis and in establishing
the standard treatment plan. [8, 9] Many recent studies have demonstrated
the presence of other prognostic factors that influence
tumors’ progress in general and oral squamous cell carcinoma in
particular such as tumor thickness or tumor depth of invasion,
tumor’s histological grade, perineural invasion, and many other
risk factors. [21-33]
Tumor thickness is considered an important risk factor for regional
lymph node involvement. [21-24] It may be of superior
influence when compared with tumor maximum dimension as
many recent trials have concluded. [10, 23, 30]
We evaluated in our study the role of tumor thickness (TT) of
oral tongue SCC in the incidence of late regional lymph node
metastasis by analyzing TT values in both studied nodal groups
(positive lymph node metastasis group and negative lymph node
metastasis group) and then statistically comparing the tumor
thickness values in our two nodal groups.
Also, we analyzed the incidence of regional nodal metastasis in
three different ranges of tumor thickness in order to detect the
thickness range that is associated with higher probability to develop
regional lymph node metastasis.
In order to lessen the misdirection in our study we exclude all
cases that is characterized with high or low tendency for nodal
involvement such T3, T4 tumors which were excluded due to the
high probability of these tumors to develop lymph node metastasis, also we exclude the tumors of histological grade III, tumors
with histologically confirmed perineural or Angio vascular invasion
and the recurrent tumors for the same aforementioned reason.
On the other hand, all cases that were diagnosed as carcinoma in
situ or exophytic type cancers were also excluded because of the
low incidence of regional nodes involvement in these kinds of
tumors.
Ultimately our study sample included 40 patients whose neck
were negative in clinical and radiological examination. Tumor
thickness in the positive nodal metastasis group ranged between
2 and 14 mm with a mean thickness of 8.96 mm, and between
1 mm and 11 mm in the negative nodal metastasis group with a
mean thickness of 4.96 mm. TT values were larger in the positive
nodal group with a significant difference as resulted in statistical
study (p value=0.006).
These results conducted with our attempt to exclude the known
risk factors that may affect regional nodal metastasis make us conclude
that thicker tongue tumors have the tendency to develop
regional nodal metastasis more than thinner tumors.
Regional nodal metastasis occurred in 14 patients of our whole
sample (35%). This percentage varies in previous studies on
OTSCC patients for many reasons relating to the inclusion criteria
of each study and the range of thickness values for their included
tumors. In a review of the incidence of late cervical nodal metastasis
in these studies, we find that the rate was 14% in the study
of Kurokawa, 28.88% in Sparano study, 47.7% in Asakage study,
19.3% in Shin study, and 43% in Yuen study. [31-35]
In our study there was a progressive increase in the rate of late
regional nodal involvement from groups of lesser thickness to
groups of larger thickness. In the group of tumor thickness
<3mm there was 18.18% of regional lymph node metastasis, with
this percentage increased to 33.33% in the group of thickness
3-6mm and 47.05% in the group>6mm thick. This increase in the
nodal metastasis incidence was expected because of the aforementioned
statistical relation between regional nodal metastasis
incidence and the increased tumor thickness values in our study
sample.
Yuen et al had recorded 8% of regional nodal metastasis for tumors’
thickness less than 3mm, 44.6% for tumors of thickness
between 3 and 9mm, and 53% for tumors’ thickness >9mm. [35]
The thickness range between 3 and 9mm was here large and needed
an equal distributions of thickness values to reach the real percentage
of metastasis incidence related to this group, because this
percentage is affected directly by the prevalent values of thickness
whether it is high or low values, in other words if high thickness
values are the dominant there will be a bias in results to more
incidence of regional node metastasis in this group, and if low
thickness values are the dominant there will be a bias in results to
less incidence of regional node metastasis in this group.
Shin et al had also studied the influence of tumor’s deep of invasion
in OTSCC patients and had recorded 7.4% of regional nodal
metastasis for tumors DOI of less than 3mm, and 23.2% for tumors
DOI of more than 3mm, [34] also here the group (>3mm)
was of large range of DOI values and again the nodal metastasis
percentage will be affected by the prevalent DOI values of the
group whether it is high or low values.
There was an agreement in previous studies that when occult
nodal involvement exceeds the percentage 20%, then neck therapy
with elective neck dissection or irradiation is indicated. [10, 20,
21] Concerning to tumors of thickness <3mm we have 18.18%
nodal involvement, this rate exceeded the critical value of 20% in
the thickness group of 3-6mm and the group >6mm with nodal
involvement rate 33.33% and 47.05% respectively, and according
to these results elective neck dissection or irradiation should be
considered for tumors of more than 3 mm thick.
Conclusion
Tumor Thickness is a predictor for regional lymph node metastasis
in oral tongue SCC patients. There was a significant incidence
of nodal metastasis in OTSCC tumors of more than 3mm thick
in comparison with tumors of less 3mm thick.
We recommend –within limits of this study- prophylactic neck
dissection or prophylactic neck irradiation and close follow-up
procedures for patients with more than 3mm thick oral tongue
SCC tumors.
Acknowledgment
Damascus University funded this study.
References
-
[1]. Sclar A. Ridge preservation for optimum esthetics and function. The Bio-Col
technique. Postgrad Dent. 1999;6(1):3-11.
[2]. Lekovic V, Camargo PM, Klokkevold PR, Weinlaender M, Kenney EB, Dimitrijevic B, Nedic M. Preservation of alveolar bone in extraction sockets using bioabsorbable membranes. Journal of periodontology. 1998 Sep;69(9):1044-9.Pubmed PMID:9776033.
[3]. Aimetti M, Romano F, Griga FB, Godio L. Clinical and histologic healing of human extraction sockets filled with calcium sulfate. International Journal of Oral & Maxillofacial Implants. 2009 Oct 1;24(5). PubmedPMID: 19865631.
[4]. Van der Weijden F, Dell'Acqua F, Slot DE. Alveolar bone dimensional changes of post-extraction sockets in humans: a systematic review. Journal of clinical periodontology. 2009 Dec;36(12):1048-58.
[5]. Sclar AG. The Bio-Col Technique. In: Soft Tissue and Esthetic Considerations in Implant Therapy. Carol Stream, IL: Quintessence Publishing Co., 2003:75-112.
[6]. Norton MR, Wilson J. Dental implants placed in extraction sites implanted with bioactive glass: human histology and clinical outcome. International Journal of Oral & Maxillofacial Implants. 2002 Mar 1;17(2).
[7]. Tonetti, MS, Hammerle, CH. European Workshop on Periodontology Group C. Advances in bone augmentation to enable dental implant placement: Consensus Report of the Sixth European Workshop on Periodontology. Journal of Clinical periodontology., 2008; 35(Suppl): 168–172. PubmedPMID: 18724849.
[8]. Chen, ST,Buser, D. Clinical and esthetic outcomes of implants placed in postextraction sites. The International Journal of Oral & Maxillofacial Implants., 2009 ;24(Suppl): 186–217. PubmedPMID:19885446.
[9]. Dohan DM, Choukroun J, Diss A, DohanSL, Dohan AJ, Mouhyi J, et al.Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral RadiolEndod., 2006; 101:e37-44. PubmedPMID:16504849.
[10]. Wang HL, Kiyonobu K, Neiva RF. Socket augmentation: rationale and technique. Implant Dentistry. 2004 Dec 1;13(4):286-96.
[11]. Gupta D, Gundannavar G, Chinni DD, Alampalli RV. Ridge Preservation done ImmediatelyfollowingExtraction using Bovine Bone Graft, Collagen Plugand Collagen Membrane. International Journal of Oral Implantology and Clinical Research. 2012 Jan 1;3(1):8-16.
[12]. Sabelman EE. Biology, biotechnology and biocompatibility of collagen. Biocompatibility of tissue analogs. 1985:21-66.
[13]. Postlethwaite AE, Seyer JM, Kang AH. Chemotactic attraction of human fibroblasts to type I, II, and III collagens and collagen-derived peptides. Proceedings of the National Academy of Sciences. 1978 Feb 1;75(2):871-5. [14]. Sakka S, Coulthard P. Bone quality: a reality for the process of osseointegration. Implant dentistry. 2009 Dec 1;18(6):480-5. Pubmed PMID: 20009601.
[15]. Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2006 Mar 1;101(3):e45-50.
[16]. Inchingolo F, Tatullo M, Marrelli M, Inchingolo AM, Scacco S, Inchingolo AD, et al. Trial with Platelet-Rich Fibrin and Bio-Oss used as grafting materials in the treatment of the severe maxillar bone atrophy: clinical and radiological evaluations. Eur Rev Med Pharmacol Sci. 2010 Dec 1;14(12):1075- 84.
[17]. Schwartz-Arad D, Chaushu G. The ways and wherefores of immediate placement of implants into fresh extraction sites: a literature review. Journal of periodontology. 1997 Oct;68(10):915-23.
[18]. Iyer S, Weiss C, Mehta A. Effects of drill speed on heat production and the rate and quality of bone formation in dental implant osteotomies. Part I: Relationship between drill speed and heat production. Int J Prosthodont. 1997; 10:411–414. Pubmed PMID:9495159.
[19]. Iyer S, Weiss C, Mehta A. Effects of drill speed on heat production and the rate and quality of bone formation in dental implant osteotomies. Part II: Relationship between drill speed and healing. Int J Prosthodont., 1997; 10:536–540. Pubmed PMID:9495174.
[20]. Tehemar S. Factors affecting heat generation during implant site preparation: a review of biologic observations and future considerations. Int J Oral MaxillofacImplants., 1999; 14:127–136. Pubmed PMID:10074763.
[21]. Garg AK. The use of osteotomes: a viable alternative to traditional drilling. Dent Implantol Update. 2002; 13:33-40. Pubmed PMID:12060956.
[22]. Saadoun AP, Le Gall MG.Implant site preparation with osteotomes: principles and clinical application. Pract Periodontics Aesthet Dent. Jun-Jul 1996;8(5):453-63.Pubmed PMID:9028267.
[23]. Yin XM, Dai JX, Wang XH, Xu DC, Zhong SZ. Observation of blood supplies system to mandible in transparent specimen. Shanghai kouqiangyixue= Shanghai journal of stomatology. 2003 Aug 1;12(4):266-8.
[24]. Hahn J. Clinical uses of osteotomes. Journal of Oral Implantology. 1999 Jan;25(1):23-9.