Latin American Countries' Regulations On Requirements Of The Installation Site, X-Ray Generating Equipment, Occupationally Exposed Worker And Radiological Protection In Dentistry
Paz Claudinne1*, Flores GirónHazaria2
1 Faculty of Dentistry, StomatologyDepartment, National Autonomous University of Honduras, Vegas del Country, #1901, Tegucigalpa, 1201, Honduras.
2 Faculty of Dentistry, StomatologyDepartment, National Autonomous University of Honduras, Tegucigalpa, 1201, Honduras.
*Corresponding Author
Paz Claudinne,
Faculty of Dentistry, StomatologyDepartment, National Autonomous University of Honduras, Vegas del Country, #1901, Tegucigalpa, 1201, Honduras.
Tel: +504 9493-6758
E-mail: claudinne.paz@unah.edu.hn
Received: July 02, 2021; Accepted: November 10, 2021; Published: November 11, 2021
Citation: Paz Claudinne, Flores GirónHazaria. Latin American Countries' Regulations On Requirements Of The Installation Site, X-Ray Generating Equipment, Occupationally Exposed Worker And Radiological Protection In Dentistry. Int J Dentistry Oral Sci. 2021;8(11):4970-4977. doi: dx.doi.org/10.19070/2377-8075-210001001
Copyright: Paz Claudinne©2021. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
The purpose of the present study is to analyze regulations of Latin American countries regarding the requirements of the installation site, X-ray generating equipment, occupationally exposed worker, and radiological protection in dentistry. AGoogle search of the Regulatory Authority´s website of each country was performed,and then the “snowball” sampling technique was carried out. The information was organized in tables according to four categories of analysis.A total of 138 regulations corresponding to 18 countries were analyzed. The regulations of Mexico, Uruguay, Dominican Republic, and Nicaragua are clear, precise, and complete because: a) they divide the requirements of the medical and dental areas, b) they detail the information they request, c) they include requirements established in the norms and recommendations of international bodies. In conclusion, in orderfor the applicant and the holder of the authorization of the installationto have a better understanding of the regulations, thesemust be clear, precise, and complete. In addition, they must contain a chapter with specific requirements for each type of X-ray generating equipment used in dentistry. Finally, they must incorporate requirements on the collaboration of the Oral and Maxillofacial Radiologist Specialist, the radiographic diagnosis, and the disposal of liquids used in the development process.
2.Introduction
3.Materials and Methods
3.Results
4.Discussion
5.Conclusion
5.References
Keywords
Latin America; Government Regulation; Dentistry; Radiology.
Introduction
In the health area, X-ray generating equipment is used to expose
the patient to ionizing radiation for justified diagnostic purposes
[1]. Since ionizing radiation is harmful to humans [1], there are
norms and recommendations of the installation site, use of X-ray
generating equipment, occupationally exposed worker (OEW),
and radiological protection. These are established by internationalorganizations
such asInternational Atomic Energy Agency
(IAEA)[2], Pan American Health Organization (PAHO) [3], and
the International Commission on Radiological Protection (ICRP)
[4, 5]. These international norms and recommendations can be
adopted by countries in their national regulations. Additionally, a
Regulatory Authority (RA) exists in each country which is defined
as an “entity legally empowered to exercise regulatory control” of
sources that emit ionizing radiation [5]. Therefore, the RA must
ensure compliance of the national regulations that control the
use and installation of X-ray generating equipment [6], as well as
grant or deny the authorizationto the interested person(s).
In relation to the international recommendations, some studies
have analyzed their implementation within the national regulations
of Korea and Ireland [7, 8]. Regarding national regulations,
the regulations of ionizing ultraviolet radiation have been analyzed
in Brazil, Europe and Canada [9-11]. In South Africa [12],
they mention some of the requirementsthey demand to the licensee
responsible for the installation (LRI) and the OEW to acquire
a license touse X-ray generating equipment. However, in the literature
no studies were found that analyze the current information
on national regulations of Latin American countries relating the requirements of the installation site, X-ray generating equipment
and radiological protection in dentistry.
The purpose of the present study is to analyze regulations of
Latin American countries on requirements of the installation site,
X-ray generating equipment, OEW and radiological protection in
dentistry.
Materials And Methods
The present study is a qualitative, diagnostic and descriptive literature
review with content analysis. The information collection
was carried out in two ways. First of all, a specific search was performed
through the Google platform to identify the RA´s website
of each Latin American country and find their national regulations.
Likewise, a general internet search was carried out to identify
the regulations of the Latin American countries that were not
found at the RA´s website. In addition, the “snowball” sampling
technique was carried out, which consisted of contacting experts
on the subject of the present studyby e-mail. The experts considered
were the representatives of the RAs of each country and also
personal contacts Specialist in Oral and Maxillofacial Radiology
(OMR) who had knowledge of the regulations of their country.
A first contact was made with the experts and if no response was
received, a second and last time was contacted. The OMRs that
responded provided general information on regulations in their
country. The RA of each Latin American country was consulted
about the validity of the regulations found through the internet
searches and the possible existence of additional regulations to
those present on their website.
The inclusion criteria used in the present study were: a) national
regulations of Latin American countries in which their official
language is Spanish or Portuguese; b) national regulations on the
requirements of the installation site, X-ray generating equipment,
OEW and radiation protection applied to dentistry, c) national
regulations that were found through the search on the Google
platform regardless of its official publication date and d) national
regulations sent by the experts via email. The exclusion criteria
were: a) requirements of the installation site and ionizing radiation
generating equipment other than X-rays, b) countries that did
not specify in their regulation or that it was not possible to verify
by the sampling technique, if the requirements they belong to a
dental or medical installationsite and c) the national regulations
that were confirmed to be repealed and/or replaced by a new
regulation.
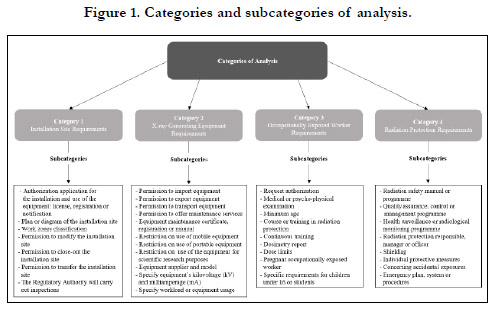
Four categories of analysis and a series of subcategories for each
of them were established. These were determined through the
reading of documents that establish the requirements considered
as objectives of the present study, these being: a) the IAEA Safety
Standards [2],b) the PAHO Recommendations [3] and c) the
ICRP Recommendations [4]. The categories and subcategories of
analysis are shown in Figure 1. Finally, the categories and subcategories
of analysis were organized in tables, showing the results
of each of the countries.
Results
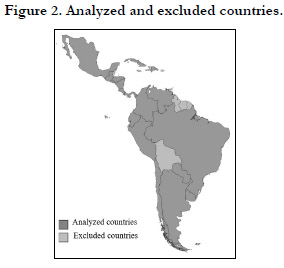
A total of 138 regulations corresponding to 18 Latin American
countries were analyzed. Figure 2 shows the analyzed countries
and excluded countries of the present study. These regulations
were included in laws, decrees, norms, agreements, resolutions,
and regulations. It alsowas found that the RA can be: a) the Ministry
of Energy, b) the Ministry of Environment, c) the Ministry
of Health, d) a dependency of the Ministries of Energy, Environment
or Health, or e) an entity created specifically.
35 experts considered for the present study were contacted by
email, of which 11 representatives from the following countries
replied: Chile, Colombia, Costa Rica, El Salvador, Honduras,
Mexico, Panama, Dominican Republic, Uruguay, Brazil and Venezuela.
In this way, it was found that in Honduras and Costa Rica
a new regulation is currently being developed, which is close to
its publication. Similarly, it was found that Argentina and Chile, in
addition to their national regulations, have specific regulations for
each region of the country.
As a general result in the present study, it was found that the 18
countries analyzed establish sanctions for non-compliance with
regulations. On the other hand, it was found that the websites of
the RAs of Argentina [13], Costa Rica [14], and Colombia [15]
have the list of people and/or companies authorized to offer
technical services, such as shielding or dosimetry.
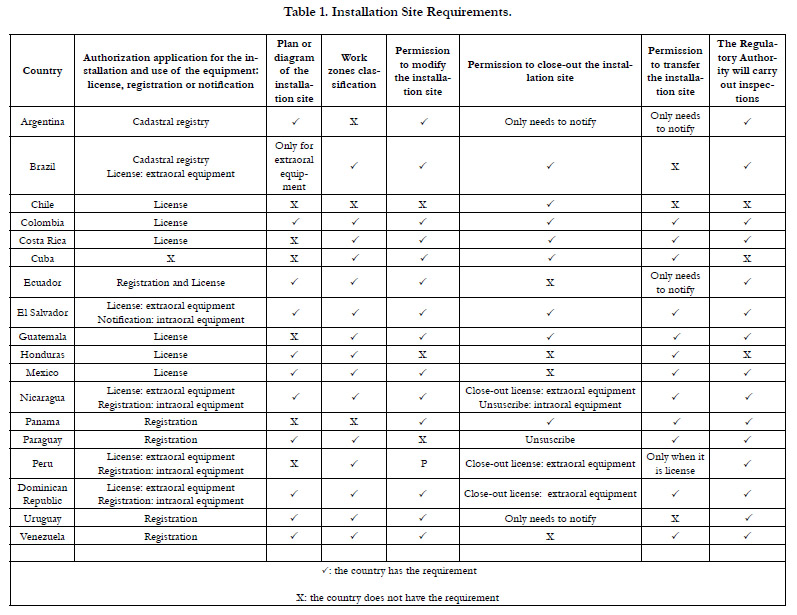
Category 1. Installation Site Requirements
Table 1 shows the installation site requirements. Regarding the
requirement of authorization application for the installation and
use of the equipment: license, registration or notification, some
countries such as El Salvador [16], Peru [17], and the Dominican
Republic [18] separate the requirements of the X-ray generating
equipment used in dentistry into categories: intraoral (or conventional)
and extraoral (or panoramic). These countries require a
license for extraoral equipment and a registration for intraoral
equipment, with the exception of El Salvador which only requires
a notification for the installation of intraoral equipment.16 However,
regardless of the separation of X-ray generating equipment
or not into categories, it was found that when a registration is
requested, the requirements are less compared to those when requesting
a license. On the other hand, Colombia [19] and Guatemala
[20] are the only countries that establish requirements for
Cone Beam Computed Tomography (CBCT), which is also one
of the equipment used in dentistry.
In addition, it was found that once the validity period of the
authorization ceases, its renewal is required in almost all of the
analyzed countries, except in Argentina, which on the RA´s website
states that the authorization is permanent as long as it is not
modified [13].
Other Installation Site Requirements
In Nicaragua21 it is established as a requirement to present the
procedures done before the purchase of a new X-ray generating
equipment. On the other hand, some countries such as Nicaragua
[21], Ecuador [22], Chile [23], Costa Rica [24] and, Honduras
[25] request a certificate or permit to operate the equipment.
Additionally, Mexico [26] establishes that the suppliers of X-ray
generating equipment must request the manufacturer or distributor
abroad to certify that the equipment meets the requirements
established in the national regulations.
Some countries such as Chile [23] and Mexico [26] request to present
an operating permit. Venezuela [27] establishes that the interested
person must present the Construction Project´s Sanitary
Conformityor the Environmental Sanitary Conformity. Argentina
[28] requests authorization from the National Atomic Energy
Commission.
Other countries such as Argentina [28] and Paraguay [29] establish
that the authorization application for the installation and use
of the equipment must be an affidavit. Guatemala [30] establishes
that when the permission to close-out the installation site is requested,
the interested person must submit an affidavit regarding
the final destination of the X-ray generating equipment. In addition,
some countries establish requirements on: a) the waiting
room [26, 27, 29, 31], b) the correct development of the radiographic
films [31-33], c) the storage area for radiographic films
[26, 27, 31, 32], d) the interpretation area [26, 31, 33],e) the toilets
for patients [27, 29, 31, 34] and f) the firefighting equipment [21,
34].
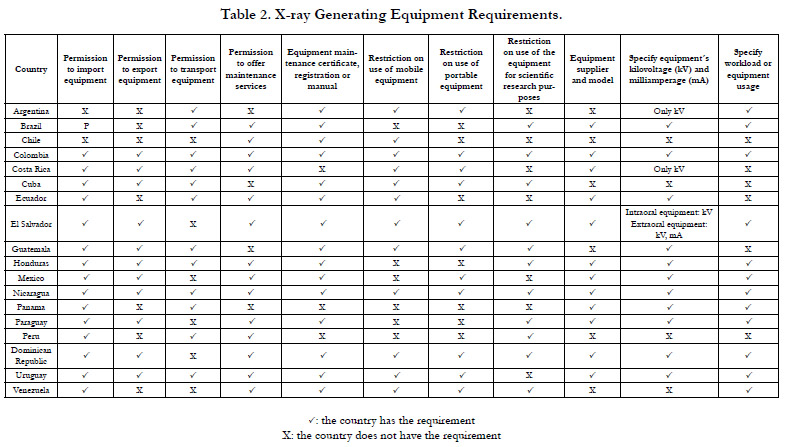
Category 2. X-ray Generating Equipment Requirements
Table 2 shows the X-ray generating equipment requirements. Regarding
the requirement of specify workload or equipment usage,
Brazil [32] and Venezuela [33] also require to keep a record of
each radiographic procedure performed. In particular, Uruguay
[31] specifies that each radiographic procedure must be registered
and archived for 5 years. Moreover, Mexico [26] establishes
that in facilities where more than 50 radiographic procedures are
performed per working day, a Quality Assurance Committee responsible
for the quality assurance programme must be created.
Argentina [34] is the only country that establishes that in dental
installations sites with exclusive dedication for radiographic procedures,
there must be an OMR.
As to the requirement of permission to offer maintenance services,
the Dominican Republic [18] establishes that the interested
person must present requirements such as: a) radiation safety
manual, b) quality assurance programme, c) assignmentof a person
responsible for protection and d) procedure manual for repair,
maintenance and calibration of the equipment.
Other X-ray Generating Equipment Requirements
Brasil [32] establishes that every six months the X-ray generating
equipment suppliers must notify the RA about the equipment
they have sold in the country.
Specifically, Venezuela [35] establishes that radiographic examinations
for theft detection purposes are considered unjustified.
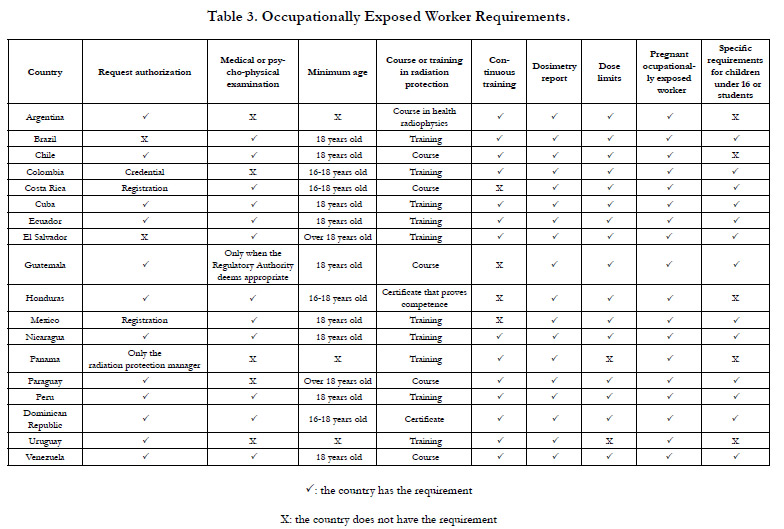
Category 3. Occupationally Exposed Worker Requirements
Table 3 shows the OEW requirements. Regarding the requirement
of request authorization, Colombia [37] establishes that the
OEW must also request a radiological protection credential from
the RA. Specifically in Cuba [38], the authorization granted to the
OEW is for life. Moreover, in some countries where the OEW
does not need to submit a request for authorization to do the work, it is the LRI that must guarantee that the OWE is qualified
to work correctly [16, 27, 32].
Regarding the requirement of medical or psycho-physical examination,
which is part of the OEW's health surveillanceprogramme,
some countries such as Cuba [36] and Brazil [32] establish
to carry them out not only at the beginning of their contract,
but also permanently.
Regarding the requirement of course or training in radiation protection,
some countries such as Peru [17], Venezuela [27] and
Mexico [39] specify that the OEW must pass a written exam on
radiation safety and protection applied by the RA. Colombia [37]
requires a written exam only to Radiology Technicians and Technologists.
Guatemala [30] requires Radiology Technicians to obtain
recognition from the Civil Association of Diagnostic Radiology
and Radiant Therapy of Guatemala in order to be eligible for
the operator's authorization.
Regarding the requirement of continuous training, some countries
establish that the LRI is in charge of facilitating this training
to the OEW [26, 32, 33]. On the other hand, in Cuba [33] and
Panama [40] it is required that it should be carried out by the radiation
protectionresponsible, manager orofficer.
Regarding the dosimetry report requirement, the LRI must keep a
record of the values indicated by the dosimeters the OEW wears
[2]. Some countries such as Paraguay [29], Venezuela [35] and
Cuba [36] establish that the LRI must keep the dosimetry report
until the OEW has 75 years of age or 30 years after he or she
ceases to work in the installation site.Moreover, Costa Rica [24]
establishes suspension of the authorization for three months to
the OEW that does not deliver its dosimeter for analysis for two
consecutive times. On the other hand, Brazil [32] establishes that
the obligation of the OEW to use a dosimeter may not be necessary
in dental installationssites with intraoral equipment that have
a workload of less than 4 milliamps per minute per week.Referring
to the requirement of dose limits, Costa Rica [24] sanctions
the OEW for exceeding the limits established in its regulation and
may even cancel the OEW license in case of receiving a second
call of attention. Ecuador establishes that the RA will notify the
LRI when the OEW has exceeded the dose limits. Consequently,
in order not to continue exceeding the dose limit, the OEW may
work at the installationsite only if the working conditions are adequate
[22].
Regarding the requirement for pregnant OEW, the LRI must
adapt the working conditions of the pregnant OEW so that during
the gestation period she is exposed as little as possibleto radiation
[22, 26, 29].
Other Occupationally Exposed Worker Requirements
Ecuador [22] and Uruguay [41] establish that the LRI must present
the obligations and tasks that each OEW performs during
their working hours. Peru [42] establishes that the OEW enjoys a
physical rest every six months in addition to their vacation period.
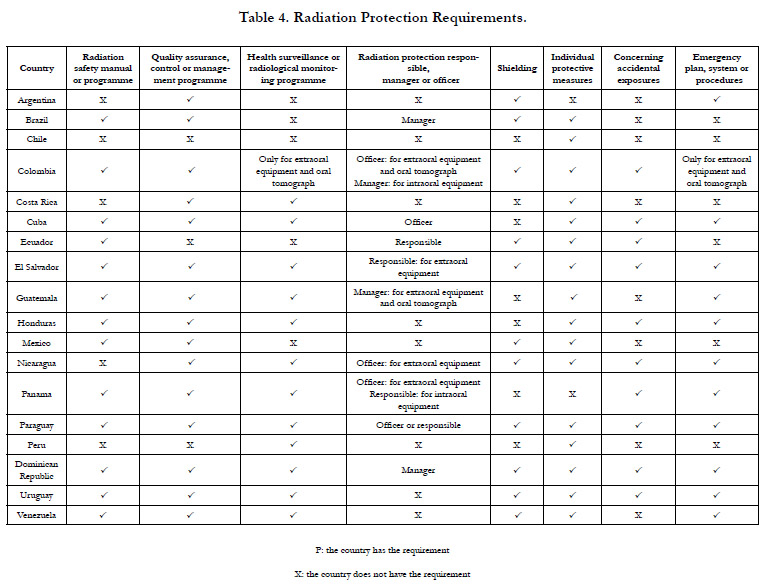
Category 4. Radiation Protection Requirements
Table 4 shows the radiation protection requirements. Regarding
the requirement of radiation safety manual or programme, the
LRI has to be in charge of its elaboration [30]. However, in Ecuador
[22] the RA made an “Instructional Form for Radiation over to the OEW.
Regarding the requirement of radiation protection responsible,
manager or officer,it was found that the requirements to be an
officer are greater compared to those required to a responsible or
manager.Additionally, Brazil [32] requires naming a responsible
technician who must also meet the requirements to perform his
specific task.In Paraguay [29], it is established that for dental installationssites
with intraoral or extraoral X-ray generating equipment,
due to justification,a responsible for radiation protection
could be namedinstead of a radiation protection officer.
In reference to the requirement of individual protective measures,
Uruguay [31]] specifies about the care of lead clothing.
Regarding the requirement concerning accidental exposures, Colombia
[19] establishes to implement the Institutional TechnovigilanceProgramme
to identify adverse events and incidents associated
with the radiological practice.
Other Radiation Protection Requirements
Some countries such as Paraguay [29], Uruguay [31], Brazil [32],
and Venezuela [35] establish that adequate protection must be
provided to those accompanying patients. Only Mexico [26] and
Dominican Republic [43] require a discard system of the liquids
used in the radiographs developing process. The regulations of
the other analyzed countries refer to the disposal of radioactive
substances, residues, waste or materials.
El Salvador [16], Uruguay [31], Brazil [32], Venezuela [33], Cuba
[36], and Colombia [37], establish that there must be an order,
request or prescription for taking a radiographic to a patient.El
Salvador [16] also determines that information management systems
must be established to guarantee the confidentiality of patient
information.
Some countries such as Argentina [13], Colombia [19], Mexico
[26], and Brazil [32] establish that a radiation physicist or consultant
specialized in radiation protection must calculate the shielding
of the installation site. On the other hand, Uruguay [31] establishes
that the protocol to be followed for each radiographic
procedure must be kept alongside the equipment control console.
Discussion
The present study is the first carried out in Latin America with the
objective of analyzing regulations of Latin American countries on
requirements of the installationsite, X-ray generating equipment,
OEW and radiological protection in dentistry.
The IAEA considers regulations to be effective only if they are
properly carried out [2]. Several studies have analyzed the role of
the RA in the regulation of radiation protection [6] and the application
of ionizing radiation regulations in medical exposure by
treating clinicians [44-46]. Regarding the analyzed regulations in
the present study, the role of the RA in each country and compliance with the requirements in accordance with the established
regulations is unknown. However, it is possible to ensure that the
analyzed regulations of Mexico, Uruguay, the Dominican Republic,
Venezuela and Nicaragua are clear, precise and complete. This
has been considered since these countries meet the following criteria:
a) the regulations divide the requirements of the medical
and dental areas, b) the requirements are explicit by detailing more
information of what they request and c) the regulations include
requirements established in the standards and recommendations
of international bodies [2-4].
It is important to note that most of the Latin American
countries´analyzed regulations do not specify the requirements
for CBCT. However, some countries use the term Extraoral
Equipment or Computed Tomography in their regulations [16,
26, 29], which could be interpreted as referring to CBCT. It is recommended
for each country to implement specific requirements
in its regulations for each type of X-ray generator equipment used
in dentistry. This is due to the fact that they have different radiation
dose values [47] and therefore require different management
and radiological protection measures.
On the other hand, it is worth mentioning that Argentina was the
only country that within its regulations requires the collaboration
of an OMR [34]. Nowadays, the OMR is recognized as a specialist
dentist in more than 50 countries. The OMR has the specific
competencies to perform correctly with the use of X-ray generating
equipment [48]. The implementation of this requirement
can improve the installation and use of X-ray generating equipment,
as well as radiation protection. Furthermore, it is important
to note that none of the Latin American countries´ regulations
analyzed refers to the mandatory radiographic interpretation in
dentistry. Radiographic interpretation is essential to determine the
clinical diagnosis and consequently the patient´s treatment [48].
Without a radiographic interpretation, valuable information on
the patient's health condition can be missed, for this reason it is
ideal to incorporate this requirement.
Also, the ICRP establishes the application of radiological and
ethical protection measures during the use of X-ray generating
equipment [5]. Regarding this, in the present study it was evidenced
that approximately half of the Latin American countries
do not establish restrictions when using mobile and/or portable
equipment and neither when it is used for scientific research purposes.
Therefore, it is essential that countries implement these
requirements in their regulations because they could be receiving
a higher dose of radiation by not taking adequate radiation protection
measures.
On the other hand, the regulations of most of the Latin American
countries analyzed tend to refer to the disposal of radioactive
substances or materials. The IAEA defines radioactive material,
substance or waste as that which has radioactivity or radionuclides
[49]. In dentistry, this type of materials, substances or waste is not
generated, on the contrary, a liquid waste with low-risk silver particle
content is generated. To dispose of this liquid waste, it is necessary
to hire an authorized company to do it [1]. In the present
study, it was found that few countries require a system to dispose
of liquids used in the radiographs developing process. Therefore,
it is recommended that Latin American countries regulations use
the correct terminology or implement this requirement.
The limitations of the present study include the access difficulty
and availability of the information on regulations of the analyzed
Latin American countries, due to the fact that only some of the
RA representatives responded to the email sent. Also, it is unknown
whether the analyzed Latin American countries implement
correctly their regulations. It is suggested that new studies
can investigate the compliance of regulations in Latin American
countries and the work of the RA regarding the issuance and control
of authorizations.
Conclusions
It is necessary for the RA of each country to present the updated
regulations on the internet to facilitate access to the information,
as well as to have digital customer service to resolve any doubts
that the applicant may have. On the other hand, for the authorization
applicant, as well as the LRI to have a better understanding
of the regulations, it is important that these are clear, precise and
complete. For this to happen, the RA must orientate in norms
and recommendations established by international bodies, as well
as request their collaboration. In addition, it is necessary that the
regulations contain a chapter with specific requirements for the
dental area, differentiating them from those of the medical area.
Similarly, they must differentiate the requirements for each type
of X-ray generating equipment used in dentistry and establish
their respective radiation protection requirements. Finally, the
regulations must incorporate requirements on the collaboration
of the OMR, on a mandatory radiographic interpretation and on
the disposal of liquids used in the radiographsdeveloping process.
Acknowledgement
We deeply thank Dr. Alejandro Hidalgo, from Chile, for the information
provided and his teachings. Likewise, to all the representatives
of the RA and the OMRs who answered our emails and provided
valuable information to carry out the present investigation.
References
-
[1]. Kasi Ganesh S, Panneerselvam E, Sharma AK, Raja Vb K. Knotless Suture
for Wound Closure in Intraoral Surgery-A Report of 2 Cases. J Oral Maxillofac
Surg. 2018 Sep;76(9):1954.e1-1954.e4. PubMed PMID: 29654780.
[2]. Selvi F, Cakarer S, Can T, Kirli Topcu SI, Palancioglu A, Keskin B, Bilgic B, Yaltirik M, Keskin C. Effects of different suture materials on tissue healing. J Istanb Univ Fac Dent. 2016 Jan 12;50(1):35-42. PubMed PMID: 28955553.
[3]. Gassner R. Wound closure materials. Oral and Maxillofacial Surgery Clinics. 2002 Feb 1;14(1):95-104.
[4]. Kasi Ganesh S, Panneerselvam E, Sharma AK, Raja Vb K. Knotless Suture for Wound Closure in Intraoral Surgery-A Report of 2 Cases. J Oral Maxillofac Surg. 2018 Sep;76(9):1954.e1-1954.e4. PubMed PMID: 29654780.
[5]. Paul MD. Bidirectional barbed sutures for wound closure: evolution and applications. J Am Col Certif Wound Spec. 2009 May 23;1(2):51-7. PubMed PMID: 24527114.
[6]. GUIDE E. Post-Operative Medications. Journal of Oral and Maxillofacial Surgery. 2015 Aug;73:1.
[7]. Greenberg JA. The use of barbed sutures in obstetrics and gynecology. Rev Obstet Gynecol. 2010 Summer;3(3):82-91. PubMed PMID: 21364859.
[8]. Ramkumar Ceyar KA, Thulasidoss GP, Raja Sethupathy Cheeman S, Sagadevan S, Panneerselvam E, Krishna Kumar Raja VB. Effectiveness of knotless suture as a wound closure agent for impacted third molar - A split mouth randomized controlled clinical trial. J Craniomaxillofac Surg. 2020 Oct;48(10):1004-1008. PubMed PMID: 32873466.
[9]. Bui CH, Seldin EB, Dodson TB. Types, frequencies, and risk factors for complications after third molar extraction. J Oral Maxillofac Surg. 2003 Dec;61(12):1379-89. PubMed PMID: 14663801.
[10]. Ferrer-Márquez M, Belda-Lozano R, Soriano-Maldonado A. Retraction Note: Use of Barbed Sutures in Bariatric Surgery. Review of the Literature. Obes Surg. 2016 Oct;26(10):2552. PubMed PMID: 27520692.
[11]. Warner JP, Gutowski KA. Abdominoplasty with progressive tension closure using a barbed suture technique. Aesthet Surg J. 2009 May-Jun;29(3):221-5. PubMed PMID: 19608073.
[12]. Rachel JD, Lack EB, Larson B. Incidence of complications and early recurrence in 29 patients after facial rejuvenation with barbed suture lifting. Dermatol Surg. 2010 Mar;36(3):348-54. PubMed PMID: 20100265.
[13]. Nett M, Avelar R, Sheehan M, Cushner F. Water-tight knee arthrotomy closure: comparison of a novel single bidirectional barbed self-retaining running suture versus conventional interrupted sutures. J Knee Surg. 2011 Mar;24(1):55-9. PubMed PMID: 21618939.
[14]. Iavazzo C, Mamais I, Gkegkes ID. The Role of Knotless Barbed Suture in Gynecologic Surgery: Systematic Review and Meta-Analysis. Surg Innov. 2015 Oct;22(5):528-39. PubMed PMID: 25320107.
[15]. Nahai F. The Art of Aesthetic Surgery: Principles and Techniques, Three Volume Set, Second Edition. CRC Press.
[16]. Sharma AK, Thulasi Doss GP, Panneerselvam E, Ganesh SK, Krishna Kumar Raja VB. Use of knotless barbed sutures for closure of intraoral incisions for maxillofacial trauma: a randomised controlled trial. Br J Oral Maxillofac Surg. 2021 Feb;59(2):e72-e78. doi: 10.1016/j.bjoms.2020.08.015. Epub 2020 Aug 14. PMID: 33384176.
[17]. Govindaraju L, Gurunathan D. Effectiveness of Chewable Tooth Brush in Children-A Prospective Clinical Study. J Clin Diagn Res. 2017 Mar;11(3):ZC31-ZC34. PubMed PMID: 28511505.
[18]. Christabel A, Anantanarayanan P, Subash P, Soh CL, Ramanathan M, Muthusekhar MR, et al. Comparison of pterygomaxillary dysjunction with tuberosity separation in isolated Le Fort I osteotomies: a prospective, multi-centre, triple-blind, randomized controlled trial. Int J Oral Maxillofac Surg. 2016 Feb;45(2):180-5. PubMed PMID: 26338075.
[19]. Soh CL, Narayanan V. Quality of life assessment in patients with dentofacial deformity undergoing orthognathic surgery--a systematic review. Int J Oral Maxillofac Surg. 2013 Aug;42(8):974-80. PubMed PMID: 23702370. [20]. Mehta M, Deeksha, Tewari D, Gupta G, Awasthi R, Singh H, Pandey P, et al. Oligonucleotide therapy: An emerging focus area for drug delivery in chronic inflammatory respiratory diseases. Chem Biol Interact. 2019 Aug 1;308:206-215. PubMed PMID: 31136735.
[21]. Ezhilarasan D, Apoorva VS, Ashok Vardhan N. Syzygium cumini extract induced reactive oxygen species-mediated apoptosis in human oral squamous carcinoma cells. J Oral Pathol Med. 2019 Feb;48(2):115-121. PubMed PMID: 30451321.
[22]. Campeau PM, Kasperaviciute D, Lu JT, Burrage LC, Kim C, Hori M, et al. The genetic basis of DOORS syndrome: an exome-sequencing study. Lancet Neurol. 2014 Jan;13(1):44-58. doi: 10.1016/S1474-4422(13)70265-5. Epub 2013 Nov 29. PMID: 24291220; PMCID: PMC3895324.
[23]. Sneha S. Knowledge and awareness regarding antibiotic prophylaxis for infective endocarditis among undergraduate dental students. Asian Journal of Pharmaceutical and Clinical Research. 2016 Oct 1:154-9.
[24]. Christabel SL, Linda Christabel S. Prevalence of type of frenal attachment and morphology of frenum in children, Chennai, Tamil Nadu. World Journal of Dentistry. 2015 Oct;6(4):203-7.
[25]. Kumar S, Rahman R. Knowledge, awareness, and practices regarding biomedical waste management among undergraduate dental students. Asian Journal of Pharmaceutical and Clinical Research. 2017;10(8):341.
[26]. Sridharan G, Ramani P, Patankar S. Serum metabolomics in oral leukoplakia and oral squamous cell carcinoma. J Cancer Res Ther. 2017 Jul- Sep;13(3):556-561. PubMed PMID: 28862226.
[27]. Ramesh A, Varghese SS, Doraiswamy JN, Malaiappan S. Herbs as an antioxidant arsenal for periodontal diseases. J Intercult Ethnopharmacol. 2016 Jan 27;5(1):92-6. PubMed PMID: 27069730.
[28]. Thamaraiselvan M, Elavarasu S, Thangakumaran S, Gadagi JS, Arthie T. Comparative clinical evaluation of coronally advanced flap with or without platelet rich fibrin membrane in the treatment of isolated gingival recession. J Indian Soc Periodontol. 2015 Jan-Feb;19(1):66-71. PubMedPMID: 25810596; PMCID: PMC4365161.
[29]. Thangaraj SV, Shyamsundar V, Krishnamurthy A, Ramani P, Ganesan K, Muthuswami M, Ramshankar V. Molecular Portrait of Oral Tongue Squamous Cell Carcinoma Shown by Integrative Meta-Analysis of Expression Profiles with Validations. PLoS One. 2016 Jun 9;11(6):e0156582. PubMed PMID: 27280700.
[30]. Ponnulakshmi R, Shyamaladevi B, Vijayalakshmi P, Selvaraj J. In silico and in vivo analysis to identify the antidiabetic activity of beta sitosterol in adipose tissue of high fat diet and sucrose induced type-2 diabetic experimental rats. Toxicol Mech Methods. 2019 May;29(4):276-290. PubMed PMID: 30461321.
[31]. Ramakrishnan M, Shukri M. Fluoride, Fluoridated Toothpaste Efficacy And Its Safety In Children-Review. International Journal of Pharmaceutical Research. 2018 Oct 1;10(04):109-14.
[32]. Gazivoda D, Pelemiš D, Vujaškovic G. A clinical study on the influence of suturing material on oral wound healing. Vojnosanit Pregl. 2015 Sep;72(9):765-9. PubMed PMID: 26554107.
[33]. Javed F, Al-Askar M, Almas K, Romanos GE, Al-Hezaimi K. Tissue reactions to various suture materials used in oral surgical interventions. ISRN Dent. 2012;2012:762095. PubMed PMID: 22645688.
[34]. Ness GM, Peterson LJ. Impacted teeth In: Miloro M, Ghali GE, Larsen PE, Waite PD, editors. Peterson’s Principles of Oral and Maxillofacial Surgery 2nd edition Ontario: BC Decker 2004; 139–156.
[35]. Gogulanathan M, Elavenil P, Gnanam A, Raja VB. Evaluation of fibrin sealant as a wound closure agent in mandibular third molar surgery--a prospective, randomized controlled clinical trial. Int J Oral Maxillofac Surg. 2015 Jul;44(7):871-5. PubMed PMID: 25721919.
[36]. Greenberg JA, Goldman RH. Barbed suture: a review of the technology and clinical uses in obstetrics and gynecology. Rev Obstet Gynecol. 2013;6(3- 4):107-15. PubMed PMID: 24920976.
[37]. McKenzie AR. An experimental multiple barbed suture for the long flexor tendons of the palm and fingers. Preliminary report. J Bone Joint Surg Br. 1967 Aug;49(3):440-7. PubMed PMID: 5341267.
[38]. Greenberg JA, Clark RM. Advances in suture material for obstetric and gynecologic surgery. Rev Obstet Gynecol. 2009 Summer;2(3):146-58. Pub- Med PMID: 19826572.
[39]. Smith K, Caceres A. Vaginal Cuff Closure in Minimally Invasive Hysterectomy: A Review of Training, Techniques, and Materials. Cureus. 2017 Oct 11;9(10):e1766. PubMed PMID: 29234570.
[40]. Krishnamoorthy B, Shepherd N, Critchley WR, Nair J, Devan N, Nasir A, Barnard JB, Venkateswaran RV, Waterworth PD, Fildes JE, Yonan N. A randomized study comparing traditional monofilament knotted sutures with barbed knotless sutures for donor leg wound closure in coronary artery bypass surgery. Interact Cardiovasc Thorac Surg. 2016 Feb;22(2):161-7. PubMed PMID: 26590381.
[41]. Siedhoff MT, Yunker AC, Steege JF. Decreased incidence of vaginal cuff dehiscence after laparoscopic closure with bidirectional barbed suture. J Minim Invasive Gynecol. 2011 Mar-Apr;18(2):218-23. PubMed PMID: 21354068
. [42]. Greenberg JA, Einarsson JI. The use of bidirectional barbed suture in laparoscopic myomectomy and total laparoscopic hysterectomy. J Minim Invasive Gynecol. 2008 Sep-Oct;15(5):621-3. PubMed PMID: 18619922.
[43]. Vijayashree Priyadharsini J. In silico validation of the non-antibiotic drugs acetaminophen and ibuprofen as antibacterial agents against red complex pathogens. J Periodontol. 2019 Dec;90(12):1441-1448. PubMed PMID: 31257588.
[44]. J PC, Marimuthu T, C K, Devadoss P, Kumar SM. Prevalence and measurement of anterior loop of the mandibular canal using CBCT: A cross sectional study. Clin Implant Dent Relat Res. 2018 Aug;20(4):531-534. PubMed PMID: 29624863.
[45]. Ramesh A, Varghese S, Jayakumar ND, Malaiappan S. Comparative estimation of sulfiredoxin levels between chronic periodontitis and healthy patients - A case-control study. J Periodontol. 2018 Oct;89(10):1241-1248. PubMed PMID: 30044495.
[46]. Ramadurai N, Gurunathan D, Samuel AV, Subramanian E, Rodrigues SJL. Effectiveness of 2% Articaine as an anesthetic agent in children: randomized controlled trial. Clin Oral Investig. 2019 Sep;23(9):3543-3550. PubMed PMID: 30552590.
[47]. Sridharan G, Ramani P, Patankar S, Vijayaraghavan R. Evaluation of salivary metabolomics in oral leukoplakia and oral squamous cell carcinoma. J Oral Pathol Med. 2019 Apr;48(4):299-306. PubMed PMID: 30714209.
[48]. Mathew MG, Samuel SR, Soni AJ, Roopa KB. Evaluation of adhesion of Streptococcus mutans, plaque accumulation on zirconia and stainless steel crowns, and surrounding gingival inflammation in primary molars: randomized controlled trial. Clin Oral Investig. 2020 Sep;24(9):3275-3280. PubMed PMID: 31955271.
[49]. Samuel SR. Can 5-year-olds sensibly self-report the impact of developmental enamel defects on their quality of life? Int J Paediatr Dent. 2021 Mar;31(2):285-286. PubMed PMID: 32416620.
[50]. R H, Ramani P, Ramanathan A, R JM, S G, Ramasubramanian A, K M. CYP2 C9 polymorphism among patients with oral squamous cell carcinoma and its role in altering the metabolism of benzo[a]pyrene. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020 Sep;130(3):306-312. PubMed PMID: 32773350.
[51]. Chandrasekar R, Chandrasekhar S, Sundari KKS, Ravi P. Development and validation of a formula for objective assessment of cervical vertebral bone age. Prog Orthod. 2020 Oct 12;21(1):38. PubMed PMID: 33043408.
[52]. Vijayashree Priyadharsini J, Smiline Girija AS, Paramasivam A. In silico analysis of virulence genes in an emerging dental pathogen A. baumannii and related species. Arch Oral Biol. 2018 Oct;94:93-98. PubMed PMID: 30015217.