Effect of Surfosept and Deconex® 53 Disinfectant Agents On The Accuracy And Dimensional Stability Of Panasil Dental Impression Materials: An In-Vitro Experimental Study
Parviz Amini1, Mostafa Alam2, Arash Ghaffarpasand3, Nasim Khaje Dalooei4, Alireza Hadi5, Kamyar Abbasi6*
1 Associate Professor, Department of Prosthodontics, School of Dentistry, Kerman University of Medical Sciences, Kerman, Iran.
2 Assistant Professor, Department of Oral & Maxillofacial Surgery, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3 Postgraduate Resident, Department of Oral & Maxillofacial Surgery, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4 Dentist, Private Practice, Tehran, Iran.
5 Assistant Professor, Department of Prosthodontics, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
6 Assistant Professor, Department of Prosthodontics, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
*Corresponding Author
Kamyar Abbasi,
Assistant Professor, Department of Prosthodontics, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Tel: 00218927980791
E-mail: kamyar.abb@gmail.com
Received: June 22, 2021; Accepted: November 07, 2021; Published: November 10, 2021
Citation: Parviz Amini, Mostafa Alam, Arash Ghaffarpasand, Nasim Khaje Dalooei, Alireza Hadi, Kamyar Abbasi. Effect of Surfosept and Deconex® 53 Disinfectant Agents On The Accuracy And Dimensional Stability Of Panasil Dental Impression Materials: An In-Vitro Experimental Study. Int J Dentistry Oral Sci. 2021;8(11):4943-4948. doi: dx.doi.org/10.19070/2377-8075-21000999
Copyright: Kamyar Abbasi©2021. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Aim and Objective: Impression materials and BegoStone casts are the main sources of cross-contamination and transmission
of dental infections between dental professionals.Considering the influence of disinfection substanceson the dimensions
of impression materials, this study was aimed to compare the effect of Surfosept and Deconex® 53 on the accuracy and
dimensional stability of dental addition siliconematerial i.e., Panasil®.
Material and Method: This in-vitro study was performed on 30 casts. The samples were divided into one control group and
two experimental groups to be disinfected with Surfosept(1%) and Deconex®53 (2%)using a sequential sampling method
(10 per group).The impressions in the experimental groups (i.e., Surfosept and Deconex®53)were rinsedand dried,thenthe
disinfectant was sprayed on the impressions and remained for 30seconds before pouring with stone. In the control group, the
impressions were onlyrinsedand dried, andwere pouredin 10 minutes. Cast dimensions were measured by a profile projector
device, and the mean values obtained from the experimental groups were compared with those of the control group.
Results: There were no significant differences among the groups regarding the height of the resulting dies without undercut
(P=0.62). Moreover, there was no significant difference among the groups regarding the distance between the two dies
(P=0.77). However, in terms of the diameter of the dies without undercut, a significant difference was observed among the
groups(P<0.005).
Conclusion: In general, no significant difference was encountered between dimensional stability and accuracy of the dental
impressions using Surfosept and Deconex®53 in this study.
2.Introduction
3.Materials and Methods
3.Results
4.Discussion
5.Conclusion
5.References
Abbreviations
Dental Disinfectants; Dental Impression Materials; Silicones.
Introduction
Dental materialsare exposed to various pathogenic microorganismswhich
are potentially harmful [1]. The main sources of
cross-contamination of dental infections include the impression
trays, dental impression materials and BegoStonecasts [2]. Chemical
disinfection has remained a common practical approach to
eliminate micro organisms, since heat or steam sterilization of
impressions and occlusal records cannot be performed due to the
risk of distortion [3]. However, as all disinfectant solutions can
have remarkable effects on the dimensional changes of impression
materials, immersion duration is recommended to be short,
i.e.less than 30 minutes [4].
There is invaluable evidence to support the transmission of microorganisms
through impression materials [5-9]. According to
the literature, most of the materials used in dental laboratories
containvarious infectious microorganisms, such as streptococci
[10, 11]. Egusa et al. estimated the prevalence of Streptococci, Staphylococcus aureus, Methicillin-resistant Staphylococcus, and
Candidain the impressions taken from the patients' mouthsat
100%, 55.6%, 25%,and 9%, respectively, [12]. Therefore, disinfection
of dental impressions can remarkably reduce the number
of bacteria and other microorganisms; however, there is no standard
method or protocolfor proper disinfection ofthe impression
materials [13].
It is of utmost importance to select the suitable material for
disinfectionand to identify the potential problems with each approach
[14-18]. Shelf life, solidification, ease of application, low
price, robustness, and resistance to different kinds of stress are
among suitable characteristics of impression materials [10, 19-
24]. On the other hand, dimensional changes of the impression
materials,following the use ofdisinfectants, are among the main
problems in the process of preparation of dental prostheses,
which could lead to treatment failure.
Silicones are the most common impression materials used to fabricate fixed
dental prosthesis [25]. Recently, the use of addition silicone impressions
is escalateddue to its high accuracy [3]. Addition-
type silicone impression materials with enhanced hydrophilic
properties have the potential to show larger dimensional changes
after disinfection, compared to conventional condensation-type
materials [26]; however, there is a dearth of research in this regard
in the literature.
Deconex® 53 is one of thematerials which iscommonly used
fordisinfecting dentalinstruments. It can be employed to eliminate
a wide range of microorganisms, including tuberculosis and viral
envelope [10]. Surfosept (Reza Rad Co, Iran)is another alcoholbaseddisinfectant
material which is used for cleaning surfaces and
objects. This substance is standardized by the European Medicines
Agency (Standards EN 1040).
The effect of Deconex on the dimensional changes of impression
materials has been investigated in some studies;however,
there is no study assessing the dimensional changes in the casts
disinfected by Surfosept material.Asdisinfecting dental impressionsis
necessary, there is a need to investigate different disinfection
materials and their effects on the characteristics ofimpression
materials,especiallytheir dimensional changes. Therefore, this
study was aimed to determine the effect of two substances (i.e.,
Surfosept and Deconex® 53) on the accuracy and dimensional
stability of dental impressionsmade of addition silicones (i.e., Panasil
®).
Materials And Methods
This in-vitro experimental study was performed on 30 casts. Samples
were divided into one control group and two experimental
groups to be disinfected by Surfosept and Deconex® 53 using a
sequential sampling method (10 per group).
Impressions
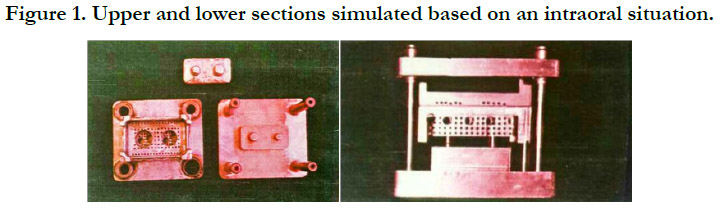
The study included two upper and lowersections simulated based
on anintraoral dentate situation (Figure 1). The lowersection had
a metal base including two stainless steel dies with three degrees
taper per each wall. One of the dieswas trimmed in a horizontal
directionat the cervical region (2 mm)to create an undercutwithdepthof
1.5mm and 45 degrees angle. The metal basehad four
guide bars to placing the upper section in an specified direction.
The die base consisted of a metal plate with dimensions of 30mm
width, 60mm height, and 15mm length. The upper section, which
acted as a custom impression tray, was made of metal base with
holesto provide retention for the impression material, andto reduce
inter-section pressure. Additionally, it had 4 holes on 4 sides
to holdthe base bars. This section had the same dimensions as the
lower section but differed in height (12mm). The die base and the
upper and lower sections were made of E.C.N, and the bars and
bushes were made of B.O.Z.
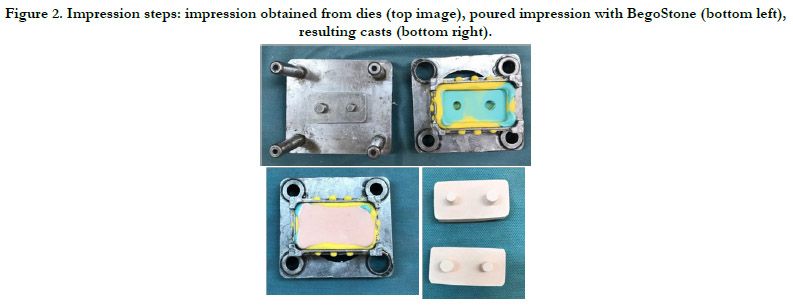
Impression process and preparation of Bego Stone Samples
In this study, two units of putty were mixed with two units ofaccelerator.
The mixture was placed in a tray and the impression was
taken. Initially, the required space for the wash layer was provided
with a 1.5 mm metal spacer. The initial setting time, working time,
and total setting time lasted 120, 120, and 240 seconds, respectively,
at 32° C.
After the solidification of the impression material, the upper and
lower sections were separated and the spacer was removed from
the putty material. Panasil®(Kettenbach Co, Germany) was injected
onthe putty material and around the die, and the impression
was taken again. After hardening, the material was separated
from the tray following the manufacturer's recommendations.
The initial setting time, working time, and thefinal setting time for
the light-bodymaterial were150, 60-90, and 240sec, respectively.
The impressions were left in the room temperature for 30 min,for
the rebound phenomenonto happen. In the next stage, the impression
was pouredusing BegoStone type IV (Wilhelm Herbst
Bremen; Germany). According to the guidelines50 gramof BegoStone
was mixed with 10cc water at 23 °Cfor 30sec, andit was
poured into the impressionin three minutes utilizingslow vibration.
The cast was separatedfrom the impression after one hour
(Figure 2).
Impression and Disinfectant Materials
Soft and lightPanasil® Putty, which areaddition-type silicone impression
materialswere employed in this study. Deconex® 53 is
adisinfectant solution, which is commonly used in hospital settings
for disinfecting flexible and rigid endoscopes. It can also be
utilized to disinfect dental instruments. The recommended concentration
of this substance is 1-2% depending on the expected
effect, while a maximum of 4% concentration is used in certain
circumstances. This solution is composed of Alkyl propylene diamine
guanidinium diacetate, and N-didecylN-methyl-poly (oxyethyl)
ammonium propionate. In this study, Deconex® 53 (1%)
was sprayed on the impressions for 30 sec.
Surfosept is analcohol-based disinfectant which can be used for
eliminating bacteria and viruses, such asinfluenza A virus subtype
H1N1, hepatitis C virus, hepatitis B virus, and human immunodeficiency
virus. This solution contains isopropanol, Didecyldimethylammonium
chloride, ethanol, and other additives.
In the experimental groups (i.e., Surfosept and Deconex® 53), the
impression materials were rinsed and dried after taking impressions.
Subsequently, the disinfectant was sprayed on the impres sions and remained for 30sec before pouring them with stone. In
the control group, the impressions were washed and dried, and
the dental impressions were poured with stone after 30sec.
A profile projector
A profile projector (Tesa Co, Switzerland) with 0.001mm resolution
was utilized to compare the dimensions and geometry of
the samples. The profile projector, which is known as an optical
comparator, is a shadow graph device which uses principles of
optics for accurate measurement and dimentional inspection of
manufactured samples.
Dimensional measurements
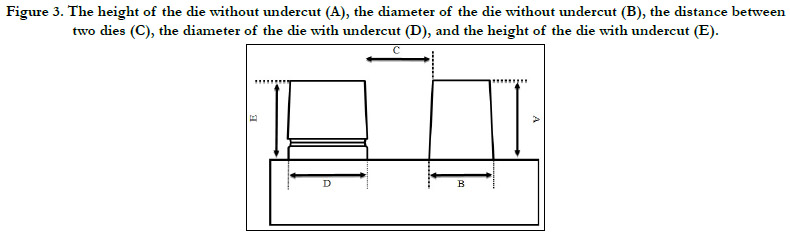
5 variables were specified and examined on cast models. These
factors which were measured by the profile projector include: the
height ofthe die without undercut (A), the diameter of the die
without undercut (B), the distance between two dies (C), the diameter
of the die with undercut (D), and the height of the die
with undercut (E) (Figure 3).
Statistical analysis
The data were analyzed in the IBM SPSS software (version 21).
The three groups were compared in terms of the mean of studied
variables. One-way ANOVAwas used to compare the groups, and
in case of a significant difference, the post hoc test (Tukey test)
was used to determine the differences among groups. A P-value
less than 0.05 was considered statistically significant.
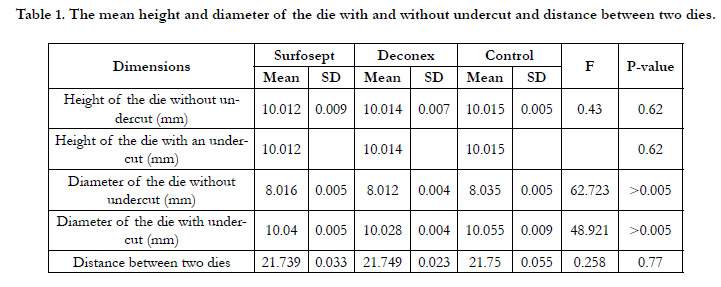
Results
The mean height and diameter of the dies with and without undercut
and distance between two diesare illustrated in Table 1.
There were no significant differences among the control and two
experimental groups in terms of the height of the dieswithout
undercut (P=0.62). Comparison of the groups regarding the
diameter of the dies without undercut showed a significant difference
among them;as a result, the experimental groups had a
lower diameter of the dies without undercut (P<0.005). No significant
difference was observed among the groups in terms of
the distance between two dies (P=0.77). In addition, regardingthe
height of the dieswith undercut, the results revealed no significant difference (P=0.62). However, there was a significant difference
among the groups regarding the diameter of the dies with undercut
(P<0.005).
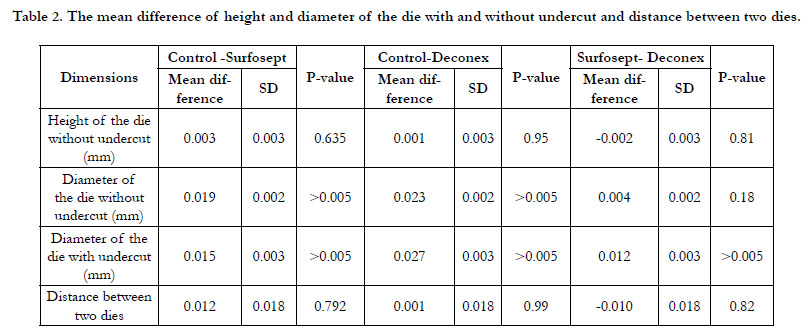
To summarize, there were significant differences between control
and two experimental groups considering the diameter of the
dieswith and without undercut. Nonetheless, no difference was
observed among the groups regarding the height of the dies with
and without undercut, and the distance between the two dies.Table
2 summarizes the results obtained from the Post hoc test.
The results obtained from this study indicated that the height of
the dies with and without undercut and the distance between two
dies had higher accuracy and dimensional stability following the
use of the disinfecting material, compared to the diameter of the
dies with and without undercut.
Figure 2. Impression steps: impression obtained from dies (top image), poured impression with BegoStone (bottom left), resulting casts (bottom right).
Figure 3. The height of the die without undercut (A), the diameter of the die without undercut (B), the distance between two dies (C), the diameter of the die with undercut (D), and the height of the die with undercut (E).
Table 1. The mean height and diameter of the die with and without undercut and distance between two dies.
Table 2. The mean difference of height and diameter of the die with and without undercut and distance between two dies.
Discussion
This study was aimed to determine the effect of two disinfectant
materials (i.e., Surfosept and Deconex® 53) on the accuracy and
dimensional changes of impression materials and the resulting
casts. The dimensional stability of the impression materials can
be influenced by several factors, including the contraction during
the polymerization, and expansion after immersion in disinfectant
solutions [27]. The thermal contraction of addition-type silicone
rubber impression materials may lead to a 10-12µm dimensional
change in cylindrical stone casts for every 1 mm increase in impression
thickness [28].
Addition silicones (i.e., Panasil®) are the impression materials
made of polyvinyl siloxane or vinyl polysiloxane. They have maximum
dimensional stability and minimum dimensional changes
when exposed to disinfectant materials [29]. The polymerization
reaction of these materials helps obtain optimal dimensional stability
[30]. Low viscosity, high hydrophilicity, excellent fluidity
with high thixotropy, and good moisture resistance are the main
characteristics of this substance leading to the increasing use of
it in recent years. Based on the obtained results, both Surfosept
and Deconex® 53 can be used without significant concern,onthe
impression materials made of Panasil®.
Hiraguchi et al. used Addition-type siliconesto assessthe dimensional
changesof impression materials after immersing in glutaraldehyde
(2%) and ortho-phthalaldehyde (0.55%)for 30 min. According
to the results of the aforementioned study, no remarkable
dimensional changes were observed in casts [28]. This findingwas
in line with the results obtained from other similar studies [31] in
which the use of addition silicone (i.e., Panasil®) was suggestedasa
suitableimpression material.
In contrast to the condensation silicones, addition silicones are
based on addition polymerization between divinylpolysiloxane
and polymethylhydrosiloxane with a platinum salt as catalyst.Addition-type silicone rubber impression materials with enhanced
hydrophilic properties contain larger quantities of surfactant,
compared to conventional materials [32]. Some evidence indicates
no change in the wettability of hydrophilic addition-type silicone
rubber impression materials after 18 hour immersion in glutaraldehyde
solution (2%); however, it is possible that the surfactant
in the impression materials leaches out rendering the impressions
less hydrophilic due to long-term immersion in disinfectant solutions.
One the other hand, the dimensions of impression may
change because of imbibition [28]. Due to the novelty of the
addition-type silicone, the type of disinfectant suitable for these
materials is not specified. Therefore, the dimensional accuracy of
the casts should be investigated in more detail.
The results obtained from this study showed no significant difference
between the experimental groups (i.e., Surfosept and
Deconex® 53) regarding the dimensional changes, except for the
diameter of the dies with and without undercut. It seems that
Deconex® 53 tends to be able to absorb water out of the air,
which is consistent to the hydrophilic property of an addition
silicone material. However, the use of Deconex® 53 with alginate
and polyether should be performed with cautionsince eliminating
microorganisms in these material could be compromised [10].
Hamedi rad et al. evaluated the dimensional stability of alginate
impressions following disinfection. The samples were divided
into four groupsto be disinfected with Sodium Hypochlorite,
Micro 10, Glutaraldehyde, and Deconex. They sprayed Micro 10
disinfectant, whereas other disinfectants were applied using immersion
technique [33]. In this study, the Deconexdis infectant
led to the maximum level of dimensional changes, which does
not comply with the findings of our study. This difference may
be due to the utilization of adifferent method for disinfection, i.e.
immersion technique, in contrast to the present study where the
spraying method was used.
In another study conducted by Ghasemi et al., Deconex, Sodium
hypochlorite (25.5%), and Epimax were utilized to disinfect impression
materials [10]. According to the results, no significant
difference was observed among the impressionsin terms of the
dimensional changes after disinfection. This finding is consistent
with the results obtained from the current study. It seems that
the use of Deconex with the spraying method hasmore benefits,
compared to the immersiontechnique. Additionally, the effect of
immersion of polyvinylsiloxane moldsin sodium hypochlorite
(5.25%) led to dimensional changes, such as shrinkage in impression
material [34].
Similarly, a study was conducted by Sabouri et al. on 30 impressions
to determine the effect of disinfectants on dimensional
changes of impression materials. In this study, 20 impressions
were disinfected by sodium hypochlorite and acid glutaraldehyde
(10 impressions per group) and 10 impressions were considered
as a control group. They rinsed impressions with cold water and
stored them at room temperature for 30 and 20 min in the disinfected
and control groups, respectively. Similar to our study, the
height and diameters of the dies and the distance between the
dies were measured in three groups. They showed no significant
differencesbetween the disinfected and control groups regarding
the dimensional changes [35].
Based on the American Dental Association guideline, the immersion
of impression materials in disinfectant solutions should not
be longer than 30 min [12]. In our study, the disinfectant solutions
were sprayed on impressions for 30 seconds. Carvalhal et
al. performed a study to determine the effects of disinfectant solutions
on the dimensional changes of the impression materials.
Four elastomeric impression materials (i.e., Xantopren, Express,
Permlastic, and Soft Impregum) were used to compare the effect
of immersion time on the dimensional changes of casts made
with each of these material. Dimensional changes were reported
in all materials over time, except for immersion periods lower
than 20 min [36]. In another study, Sodium hypochlorite (25.5%),
Deconex, and Sanosil were employed for disinfecting impressionsfor8-
10 min periods, and the results were acceptable in terms
of accuracy and dimensional stability of the material [10].
Conclusions
Based on the obtained results, no difference was reported between
the experimental and control groups regarding the height of the
dies without undercut. In addition, no difference was reported
between the two experimental groups and the control group in
terms of the distance between two dies. However, a difference
was observed between the experimental and control groups in
terms of the diameter of the dies both with and without undercut.
In general, no significant difference was observed between
Surfosept (1%) and Deconex® 53 (2%) in terms of influencing
the dimensions or accuracy of the impression materials.
Acknowledgments
The authors would like to thank the authoritiesat Kerman Dental
School, Kerman University of Medical Sciences, Kerman, Iran,
for their cooperation inconducting this study.
References
-
[1]. Aroori S, Spence RA. Carpal tunnel syndrome. Ulster Med J. 2008
Jan;77(1):6-17. PubMed PMID: 18269111.
[2]. Haghighat A, Khosrawi S, Kelishadi A, Sajadieh S, Badrian H. Prevalence of clinical findings of carpal tunnel syndrome in Isfahanian dentists. Adv Biomed Res. 2012;1:13. PubMed PMID: 23210072.
[3]. Bland JD. Carpal tunnel syndrome. BMJ. 2007 Aug 18;335(7615):343-6. PubMed PMID: 17703044.
[4]. Alhusain FA, Almohrij M, Althukeir F, Alshater A, Alghamdi B, Masuadi E, Basudan A. Prevalence of carpal tunnel syndrome symptoms among dentists working in Riyadh. Ann Saudi Med. 2019 Mar-Apr;39(2):104-111. Pub- Med PMID: 30905925.
[5]. Khosrawi S, Maghrouri R. The prevalence and severity of carpal tunnel syndrome during pregnancy. Adv Biomed Res. 2012;1:43. PubMed PMID: 23326774.
[6]. Oktayoglu P, Nas K, Kilinç F, Tasdemir N, Bozkurt M, Yildiz I. Assessment of the Presence of Carpal Tunnel Syndrome in Patients with Diabetes Mellitus, Hypothyroidism and Acromegaly. J ClinDiagn Res. 2015 Jun;9(6):OC14-8. PubMed PMID: 26266148.
[7]. Ahmed A, Khan A, Siddiqui Z, AHMED MR, Askari H, Zahid A. Prevalence of carpel tunnel syndrome in the dentists working in Karachi. Pakistan Oral & Dental Journal. 2014 Dec 1;34(4).
[8]. Chin DH, Jones NF. Repetitive motion hand disorders. J Calif Dent Assoc. 2002 Feb;30(2):149-60. PubMed PMID: 11883427.
[9]. Brown PN. What's ailing us? Prevalence and type of long-term disabilities among an insured cohort of orthodontists. Am J OrthodDentofacialOrthop. 2004 Jan;125(1):3-7. PubMed PMID: 14718873.
[10]. BorhanHaghighi A, Khosropanah H, Vahidnia F, Esmailzadeh S, Emami Z. Association of dental practice as a risk factor in the development of carpal tunnel syndrome. J Dent (Shiraz). 2013 Mar;14(1):37-40. PubMed PMID: 24724115.
[11]. Inbasekaran D, Sankari M, Nambi SG. Prevalence of carpal tunnel syndrome among dentists in Chennai, India. Drug Invention Today. 2018 Nov 2;10(3):3262-5.
[12]. Lalumandier JA, McPhee SD. Prevalence and risk factors of hand problems and carpal tunnel syndrome among dental hygienists. J Dent Hyg. PubMed PMID: 11475758.
[13]. Leggat PA, Kedjarune U, Smith DR. Occupational health problems in modern dentistry: a review. Ind Health. 2007 Oct;45(5):611-21. PubMed PMID: 18057804.
[14]. Munirah MA, Normastura AR, Azizah Y, Aziah D. Prevalence of probable carpal tunnel syndrome and its associated factors among dentists in Kelantan. International Journal of Collaborative Research on Internal Medicine & Public Health. 2014;6(8).
[15]. Ehsan M, Ehsan S, Arshad H. Frequency of carpal tunnel syndrome in dentists working in government hospitals of lahore. Int J Sci Res. 2013;14:2319- 7064.
[16]. SayeghGhoussoub M, Ghoussoub K, Moucharrafieh L, Khoury A, Sleilaty G, Rifaï K. Troubles musculo-squelettiques chez une population de chirurgiens- dentisteslibanais: Fréquence et facteurs de risque [Musculo-skeletal problems among Lebanese dental surgeons. Occurrence and risk factors]. J Med Liban. 2005 Jan-Mar;53(1):21-7. PubMed PMID: 16398209.
[17]. oic L, Bojnec V, Lonzaric D, JesenekPape B. An advanced stage of carpal tunnel syndrome - is night-time splinting still effective? Int J Occup Med Environ Health. 2020 Oct 20;33(6):771-780. PubMed PMID: 32929289.