Tannerella Forsythia in Oral Squamous Cell Carcinoma - An Exploratory Study
Sriram Kaliamoorthy1*, R Saranyan2, Jeyakumar Balakrishnan3
1 Associate Professor, Vinayaka Mission’s Medical College and Hospital, Vinayaka Mission’s Research Foundation (Deemed to be University), Karaikal, Puducherry, India.
2 Professor, Vinayaka Mission’s Sankarachariyar Dental College, Vinayaka Mission’s Research Foundation (Deemed to be University), Salem, Tamilnadu, India.
3 Central Research Laboratory, Vinayaka Mission’s Medical College and Hospital, Vinayaka Mission’s Research Foundation (Deemed to be University), Karaikal, Puducherry, India.
*Corresponding Author
Sriram Kaliamoorthy,
Associate Professor, Vinayaka Mission’s Medical College and Hospital, Vinayaka Mission’s Research Foundation (Deemed to be University), Karaikal, Puducherry, India.
Tel: +91-9597889524
E-mail: ksrirammds@gmail.com
Received: October 05, 2021; Accepted: October 22, 2021; Published: October 30, 2021
Citation: Sriram Kaliamoorthy, R Saranyan, Jeyakumar Balakrishnan. Tannerella Forsythia in Oral Squamous Cell Carcinoma - An Exploratory Study. Int J Dentistry Oral Sci. 2021;8(10):4873-4875. doi: dx.doi.org/10.19070/2377-8075-21000985
Copyright: Sriram Kaliamoorthy©2021. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: While no single species has been implicated as the primary pathogen and the available evidence is consistent
with a polymicrobial disease etiology, the red-complex bacteria consisting of Porphyromonasgingivalis, Treponemadenticola
and Tannerella forsythia has been strongly implicated in the onset of periodontitis a chronic inflammatory disease affecting
the supporting tissues of the teeth in the oral cavity. Chronic inflammation is believed to act as catalyst for tumour development
and progression.
Objective: To study the presence ofTannerella forsythia in the tissue samples oforal squamous cell carcinoma.
Materials and Methods: Oral squamous cell carcinoma (n= 30) and oral mucosal non-cancerous tissue specimens (n=30)
were obtained from patients and controls separately. RNA was isolated from each aseptically deliquesced tissue specimen
and transformed to cDNA using the Trizol technique. Specific gene amplification of Tannerella forsythia was done byusing
synthesized cDNA as template for PCR reaction.
Results: Tannerella forsythia was detected in 5 oral squamous cell carcinoma tissuesamples and was not found in any of the
control tissue samples.
Conclusion: Tannerella forsythia could play a role in the pathogenesis of oral squamous cell carcinoma.
2.Introduction
3.Materials and Methods
3.Results
4.Discussion
5.Conclusion
5.References
Keywords
Tannerella Forsythia; Oral Malignancy; Gene Amplification.
Introduction
Oral squamous cell carcinoma (OSCC), a subset of Head and
neck squamous cell carcinoma (HNSCC), is the most common
malignant oral neoplasm. OSCC accounts for 90% of all oral
malignancies, and it has a poor 5-year survival rate that has not
changed in decades. As risk factors, smoking, alcohol consumption
and human papilloma virus (HPV) infection have been implicated.
However, these risk factors alone have not been sufficient
in explaining the incidence and aggressive behaviours of OSCC
[1]. Thus, other factors, such as oral dysbiosis may play an important
role in OSCC tumor development, progression and metastasis,
yet this has not been well explored. Indeed, dysbiosis of
the commensal oral microbiota and their subsequent invasion of
the tooth supporting structures (e.g., the gingiva, periodontal ligament
and bone) lead to the initiation and propagation of an inflammatory
condition termed periodontitis or periodontal disease
(PD) [2]. Individuals diagnosed as periodontitis were 3.7 times to
develop OSCC, indicating that periodontitis was one of the risk
factors of oral cancers. Our previous study found that periodontal
pathogens, Porphyromonas gingivalis, and Treponema denticola,
were prevalent in OSCC.
Treponema denticola, Porphyromonas gingivalis and Tannerella forsythia
appears in late stages of oral biofilm development and comprises
the bacterial “red complex” that is considered pathogenic in the etiology of periodontal disease. Other periodontopathogenic bacteria
have been proposed for inclusion in the red complex [3].
Tannerella Forsythia is a Gram-negative, anaerobic bacterium.
Described by Tanner and co-workers, it was referred to as Bacteroidesforsythus.
Currently, it belongs to the genus Tanerella. The
bacteria’s breeding is not easy due to its demanding growth conditions
[4]. Recently, mounting evidence suggests a causal relationship
between Tannerella forsythia infection and the development of
malignancies. Herein we report the presence of Tannerella forsythiain
the tissue of OSCC based on PCR based amplification.
Materials and Methods
Patients and Tissues samples
There were 60 tissue samples collected in this study, 30 of which
were from oral squamous cell carcinomas and 30 of which were
from normal oral cavities. Prior to the initiation of the study. Institutional
ethicalclearance was acquired. Tissue samples were collected
after informed consent was obtained from the patients and
control subjects. Both tumour and control tissues were washed
twice with sterile 1X PBS (Phosphate Buffered Saline) before being
transferred to a 2 mL microfuge tube containing Trizol reagent
and kept at -20°C until further use.
Total RNA isolation from tissue
RNA was isolated using Trizol based RNA isolation procedures.
From frozen tissue, 20 µm sections were made and collected in a
frozen tube. Approximately 250 mg tissue material was dissolved
in 5 ml Trizol(Thermo Fisher -India) and homogenised with amicropestle
for one minute. After adding 1 ml chloroform and mixing
for one minute, the suspension was centrifuged at 12, 000 rpm
for 10 minutes. A second phenol/chloroform extraction was performed,
followed by an isopropanol precipitation. The air-dried
pellet was dissolved in 100 µl double distilled water and further
purified with a RNeasy mini column (Qiagen GmbH, Hilden, Germany),
according to the manufacturer's instructions.The isolated
RNA (1 µg/lane) was electrophoresed for three hours at 50 V on
a 1% agarose/formalin gel, and stained with ethidium bromide to
assess the quality of the RNA.The concentration of isolated total
RNAs was determined by measuring absorbance values from
wavelengths at 230, 260 and 280 nm using the NanoDrop 2000
spectrophotometer (Thermo Fisher Scientific, Waltham, USA).
Purity of the total RNA was estimated by calculating the A260/
A230 and A260/A280 ratios to evaluate the levels of protein and
polysaccharide/phenolic compound contamination, respectively.
cDNA synthesis
First-strand of cDNA was synthesized with total RNAs (2.5 µL)
by using cDNA Synthesis kit (Thermo Scientific RevertAid First
Strand).
Amplification and identification of Tannerella forsythia specific
gene
PCR was performed with Taq polymerase master mix (Ampliqon,
Odense, Denmark). The following thermocycling parameters
were used during for PCR analyses. These were an initial denaturation
at 94 °C for 30 s, followed by 34 cycles of 94 °C denaturation
for 15 s, 56 °C annealing for 35 s and 72 °C extension
for 30 s, a final extension step at 72 °C for 10 min and an infinite
hold at 10 °C. The resulting PCR products were then examined by
electrophoresis in 1% agarose gel.
Copy number quantification
Gel snaps were processed with Image J, which turned the pixel
concentrations of the bands into pixel intensity ratios. Because
the stain binds more strongly to larger fragments than to smaller
fragments, it was required to compensate for them by utilizing the
stain. Correction values were calculated in order to quantify the
amount of DNA present in each band. By staining the molecular
weight markers, which had been separated on agarose and dyed
with Orange G, the correction factor was established. These parameters
were used to compare an experimental value to a forecasted
value.
Results
Tissue samples from 20 male and 10 female volunteers with
various periodontal diseases were used to investigate the prevalence
of Tannerella forsythia. As a control, the same numbers of
healthy tissues were employed. The average age of the patients
was 55 for men and 47 for women. The template DNAused for
the PCR experiment to detect Tannerella forsythiayielded a specified
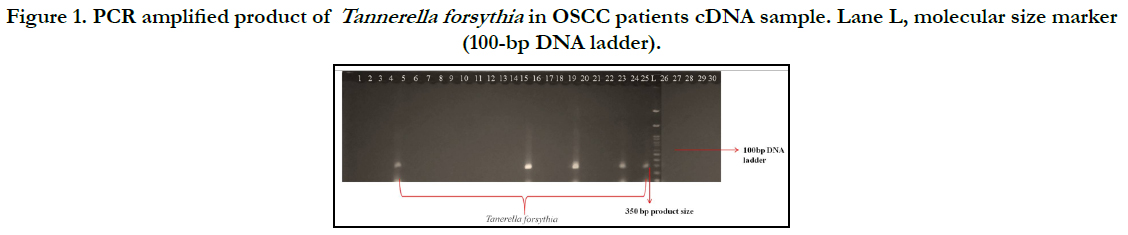
amplicon. Tannerella forsythia was detected in 5 (16.6%)
among 30 subjects.For the positive control reactions, each reaction
produced a single band of the predicted size, as shown in
Figure 1. Tannerella forsythia was found to be positive in five oral
squamous cell carcinoma tissue samples, indicating that the bacterium
may have an association to oral squamous cell carcinoma.
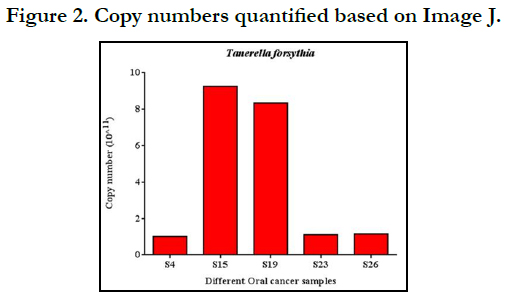
Copy number quantification
By analyzing the band intensities with Image J software, we were
able to quantify the copy numbers, as shown in Fig. 2. In all the 5
samples, the copy numbers were more than8 *10.
Figure 1. PCR amplified product of Tannerella forsythia in OSCC patients cDNA sample. Lane L, molecular size marker (100-bp DNA ladder).
Discussion
Oral cavity is one of the well-studied microbiomes till date with
a total of 392 taxa that have at least one reference genome and the total genomes across the oral cavity approaching 1500 [5].
Tannerella forsythia has been found to be associated with an increased
risk of oral cancer and holdsnumber of signalling pathways
responsible for cancer development. Tannerella forsythia yield
several virulence factors and immune evasion factors, instigating
inflammation and destruction of periodontal tissues [6-8]. The
multifactorial aetiology of cancer formation should also be considered,
and the role of bacteria should be seen as a key, but not
the most important, aspect in this regard.In this current study,
5 patients were showed positive for the presence of Tannerellaforsythia
in oral cancer tissue, and we summarized the associations
between these bacteria and incidence and prognosis of oral
cancer. Moreover, there is extensive evidence showing that Tannerella
forsythia and Porphyromonas gingivalis are abundant in
tumors and activate transduction pathways, such as anti-apoptotic
pathway and nuclear factor-?B, leading to prognosis of cancer.
Improving oral hygiene and treatment of periodontitis can significantly
reduce the occurrence of oral cancer.
References
-
[1]. Mehrotra R, Yadav S. Oral squamous cell carcinoma: etiology, pathogenesis
and prognostic value of genomic alterations. Indian journal of cancer. 2006
Apr 1;43(2):60.Pubmed PMID: 16790942.
[2]. Dahlen G, Basic A, Bylund J. Importance of virulence factors for the persistence of oral bacteria in the inflamed gingival crevice and in the pathogenesis of periodontal disease. Journal of clinical medicine. 2019 Sep;8(9):1339. Pubmed PMID: 31470579.
[3]. Bodet C, Chandad F, Grenier D. Pathogenic potential of Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia, the red bacterial complex associated with periodontitis. Pathologie-biologie. 2006 Oct 17;55(3-4):154-62. Pubmed PMID: 17049750.
[4]. Sharma A. Virulence mechanisms of Tannerella forsythia. Periodontology 2000. 2010 Oct;54(1):106.Pubmed PMID:
[5]. Deo PN, Deshmukh R. Oral microbiome: Unveiling the fundamentals. Journal of oral and maxillofacial pathology: JOMFP. 2019 Jan;23(1):122.
[6]. Jun HK, Jung YJ, Choi BK. Treponema denticola, Porphyromonas gingivalis, and Tannerella forsythia induce cell death and release of endogenous danger signals. Archives of oral biology. 2017 Jan 1;73:72-8.
[7]. Shimotahira N, Oogai Y, Kawada-Matsuo M, Yamada S, Fukutsuji K, Nagano K, Yoshimura F, Noguchi K, Komatsuzawa H. The surface layer of Tannerella forsythia contributes to serum resistance and oral bacterial coaggregation. Infection and immunity. 2013 Apr;81(4):1198-206. Pubmed PMID:23357386.
[8]. Sharma A. Persistence of Tannerella forsythia and Fusobacterium nucleatum in Dental Plaque: a strategic alliance. Current Oral Health Reports. 2020 Mar;7(1):22-8.