Effectiveness of a Novel Nano-Silver Fluoride with Green Tea Extract Compared with Silver Diamine Fluoride: A Randomized, Controlled, Non-Inferiority Trial
Zuhair Al-Nerabieah1*, Ettihad Abo Arrag2, John C Comisi3, Anas Rajab4
1 Pediatric dentistry department, Dental Collage, Damascus University, Al-Mazzeh St. Damascus, Syria.
2 Senior lecturer, Pediatric dentistry department, Dental Collage, Damascus University, Al-Mazzeh St. Damascus, Syria.
3 Associate professor, Restorative Dentistry, Department of Oral Rehabilitation, Medical University of South Carolina, James B. Edwards College of Dental Medicine, Charleston, South Carolina, USA.
4 Associate professor, Organic chemistry department, Faculty of Pharmacy, Syrian private University (SPU), Al-Mazzeh St. Damascus, Syria.
*Corresponding Author
Zuhair Al-Nerabieah,
Pediatric dentistry department, Dental Collage, Damascus University, Al-Mazzeh St. Damascus, PO Box 30621, Syria.
Tel: +963969960118
E-mail: zuhairmajid@gmail.com
Received: May 29, 2020; Accepted: June 22, 2020; Published: June 26, 2020
Citation:Zuhair Al-Nerabieah, Ettihad Abo Arrag, John C Comisi, Anas Rajab. Effectiveness of a Novel Nano-Silver Fluoride with Green Tea Extract Compared with Silver Diamine Fluoride: A Randomized, Controlled, Non-Inferiority Trial. Int J Dentistry Oral Sci. 2020;7(6):753-761. doi: dx.doi.org/10.19070/2377-8075-20000148
Copyright: Zuhair Al-Nerabieah©2020. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
The aim of this study wasto evaluate whether the cariostatic efficacy of a biologically synthesized novel Nano-Silver Fluoride with green tea extract (NSF-GTE) is non-inferior to Silver Diamine Fluoride (SDF) 38% in deciduous teeth in preschool children. This study was a randomized, single-blinded, non-inferiority clinical trial. Sixty-three preschoolers with a total of 164 active lesions were selected and randomly assigned into two groups (A: 32 children with 83 lesions treated with NSF-GTE - B: 31 children with 81 lesions treated with SDF). Clinical evaluation was performed at 21 days,3 and 6 months after treatment using International Caries Detection and Assessment System (ICDAS II) criteria to assess carious lesions activity. Non- Inferiority margin was set at 15%. At six months, Total arrest rate was 67.4% and 79.6% for NSF-GTE and SDF respectively (P> 0.05). Furthermore, 95% confidence interval of the Relative Risk for (group A) at the three follow-up periods lies entirely below the predefined margin in comparison to (group B). Also, it was observed that anterior teeth and single surface lesions had higher arrest rates as compared to posterior teeth and multiple surface lesions (P<0.05). Non-Inferiority was demonstrated, and both SDF and NSF-GTE presented cariostatic efficacy in primary teeth.
2.Introduction
3.Materials and Methods
4.Results
5.Discussion
6.Conclusions
7.Author Contributions
8.Funding
9.Acknowledgments
10.References
Keywords
Nano-Silver; Fluoride; Green Tea; Silver Diamine Fluoride; Caries; Children.
Introduction
Despite the advanced milestones that have happened in preventive dentistry in the last two decades, tooth decay still a ubiquitous chronic disease around the world [1]. An enormous amount of dental caries lesions is left untreated since oral health care demands are beyond the capacities of the dental health care organizations [2]. In most deprived populations, this results in significant disease advancement that leads to ache, higher expenses, and decreased quality of life for the affected children and their families [3]. The development and implementation of cariostatic agents in deprived populations have been demanded by investigators and clinicians [4].
Recently, Silver Diamine Fluoride (SDF) has been demonstrated
by various investigators, to be effective in arresting dentin carious lesions. However, the application of SDF creates dark black staining
of caries tissues, which can be a significant drawback of its
use, especially on anterior teeth [5].
Antimicrobial efficacy of Nano-Silver particles (AgNPs) against
cariogenic bacteria have been demonstrated in-vitro studies [6].
Dos Santos et al. investigated the clinical efficacy of a chemically
synthesized Nano Silver Fluoride (NSF). Even though it was effective
in arresting dentin lesions without causing black stains [4],
there is a concern regarding the hazardous chemical agents used
as a reducing agent, and this has drawn attention to the safety of
this product [7].
In contrast, Biological synthesis approach utilizing plant extracts
have emerged as a simple alternative to typical chemical synthesis
techniques [8]. The use of a green tea extract has recently become of great interest due to its favorable protocol, which is a fast,
simple, eco-friendly, safe, and economically feasible green technique
[9, 10]. Green tea (Camellia Sinensis) is mainly consisted of
polyphenolic compounds that act as a reducing and capping agent
in the biological synthesis process of AgNPs [11].
To the extent of our knowledge, after reviewing the literature,
there were no in-vivo studies that have evaluated the efficacy of
biologically synthesized Nano-Silver Fluoride in arresting caries.
Thus, this study aim was to investigate whether the cariostatic
efficacy of a biologically synthesized novel Nano-Silver Fluoride
with green tea extract (NSF-GTE) is non-inferior to Silver Diamine
Fluoride (SDF) 38% in primary teeth in preschool children.
Materials and Methods
Chemical substances were acquired from Sigma Aldrich (St. Louis,
MO, USA), and green tea leaves purchased from a local market.
Deionized water was prepared using a water deionizer machine
(ZHUOYUE-1, Sichuan, China). Silver Diamine Fluoride 38%
was obtained from Elevate Oral care (Advantage Arrest®, Elevate
Oral Care LLC., West Palm Beach, FL, USA).
“Green tea extract was prepared by exactly weighting 1.3 g of green tea leaves, which was transferred into a 250 ml conical flask, already containing 100 mL of deionized water. The mixture was then heated at 80 °C for 20 min, and the extract was cooled at room temperature and then filtered by using Whatman® filter paper grade 1 (Healthcare GE, Boston, USA)”. The filtered extract was used at the same session to synthesize Silver Nanoparticles [12].
“60 ml of
green tea extract was taken via pipette and added to 500 ml of
deionized water in a volumetric flask and hand-shaken to homogenize
the dilution. The resulting pH of the solution was six and
was further modified to a pH of 10 through the addition of potassium
carbonate (K2CO3). 20 ml of the 1mM AgNO3 aqueous
solution was then added in a single shot.” [12].
The formation of AgNPs was observed by the change of green
tea extract color from yellow to dark brown after adding Silver
Nitrate, which was reported in other studies [8, 9, 12].
At the end of the biological synthesis of Ag-NPs, Sodium Fluoride
(NaF) was added to improve the stability and the cariostatic
efficacy of the solution (10104 ppm) [4]. The final solution (NSFGTE)
was stored in a dark amber bottle at (4C°) until further use.
The morphology of NSFGTE
was analyzed using Scanning Electron Microscopy (SEM)
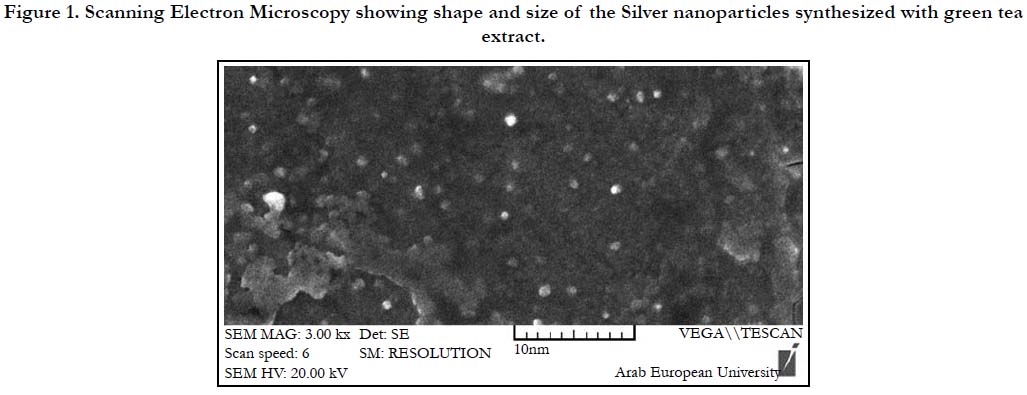
(VEGA II, TESCAN, CZE). SEM analysis showed that AgNPs
were spherical with an average particle size of 4 nm. (Figure 1)
Figure 1. Scanning Electron Microscopy showing shape and size of the Silver nanoparticles synthesized with green tea extract.
This clinical study design was a randomized, single-blind, activecontrolled,
parallel-group, non-inferiority trial. The study was
conducted in accordance with the Declaration of Helsinki and
ethical approval was obtained from the Institutional Review
Board in Damascus University (R.N 2297). Also, the trial was
registered in the Australian New Zealand Clinical Trial Registry
(ANZCTR) (Trial Id: ACTRN12618001865202). The extension
of the CONSORT statement for reporting non-inferiority trials
was applied in this study [13].
The study was conducted between 2018 and 2019 in a single kindergarten
in Damascus where the water supply has a very low
concentration of Fluoride 0.03 ppm [14].
The sample size was calculated with G*Power 3.1.9 software
(University of Kiel, Germany). The significance level was set at
0.05, the statistical power was set at 80% and the predefined noninferiority
margin of carious lesions arrest was set at 15%. Based
on a student t-test and previous studies [4], an estimated 60 carious
lesions in each group were required to demonstrate an effect
size (0.4) in the average proportion of arrested caries. The total
sample size was raised by 20% to avert the adverse effect of the
possible drop rate.
A single Investigator screened 150 children for eligibility, depending
on the following inclusion and exclusion criteria.
1. Healthy children willing to co-operate.
2. aged 36 to 60 months
3. Have not been treated with antibiotics in the past month before enrollment to avoid the possible hyposalivation effect [15].
1. Each participant had at least one active lesion with dentin exposed
based on the ICDAS II (“Code 5: dentin cavity easily visible
with the naked eye where the surface of cavity feels soft or
leathery on gentle probing”) [16].
1. Children weighing less than 15kg [17].
2. known sensitivity to Silver or other heavy-metal ions [18].
3. presence of gingivitis.
4. The tooth was excluded if it had pulp exposure, abscess/fistula,
sensitivity to percussion or discoloration related to tooth nonvitality.
Written informed consent and an invitation letter that describes
the scope and purpose of the research was sent to parents and
guardians of eligible children. Children who were enrolled in this
study had brought back a signed consent before the initiation of
the trial.
Children in the kindergarten were supplied with written instructions
for maintaining oral hygiene, toothbrush and a fluoridated
toothpaste containing 600 ppm of Fluoride.
Sixty-three children with a total of 164 active caries lesions met
the inclusion criteria and were enrolled in this study. Each participant
was assigned to one treatment group to avoid the possible
cross-over effect of the NSF-GTE and SDF. Hence, children
were allocated into two strata based on their carious lesion number
(1-2 lesions or ≥3 lesions).
Then, Statistical Package for the Social Sciences (SPSS) version
25.0 (IBM, Chicago, IL, USA) was utilized to generate a randomization
schedule with stratification. After that, Children were
randomly assigned to one of the groups with 1:1 ratio. This Randomized
allocation process was performed by an external investigator
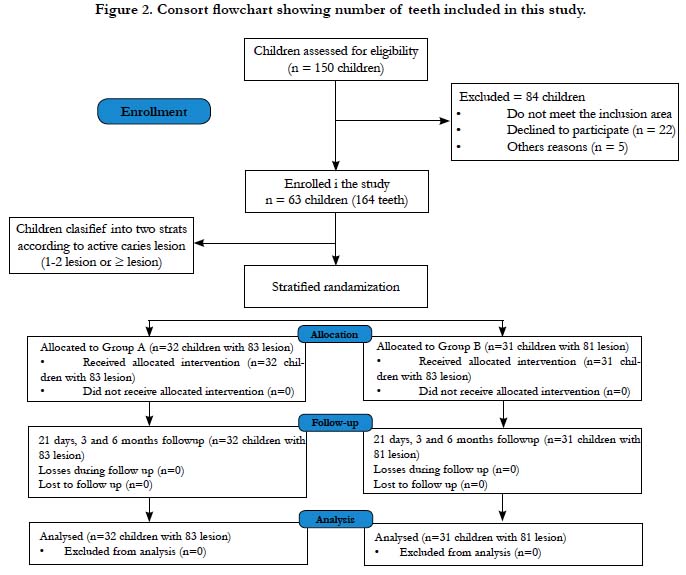
to achieve allocation concealment. The Consort diagram
that describes participant flow is outlined in Figure 2.
Clinical examination was carried out by a single experienced investigator
(AZ) using a disposable blunt probe and mirror. In the
first session, decay, extracted due to caries, filled tooth (deft) index
was scored for enrolled children to evaluate oral hygiene status.
Then, caries activity assessment was carried out using visual and
tactile inspection, according to ICDAS II. Teeth with easily detectable
cavitated lesions, where the surface felt soft or leathery
on gentle probing, were considered active cavitated lesion and
enrolled in the study.
A total of 63 children with 164 lesions were stratified according to
active carious lesions (1-2 lesions or ≥ 3 lesions), then they were
randomly allocated into one of the following groups:
• Group A: Nano-Silver Fluoride with green tea extract (NSFGTE)
• Group B: SDF
In the next visit, the affected tooth surfaces were gently cleaned
by a disposable micro-brush applicator for at least 30 seconds
and then dried with cotton gauze sponges. No effort was made
to remove the carious tissue before the application of the agent.
Gingival tissues of the targeted tooth were protected with petroleum
jelly, and isolation was carried out using cotton rolls.
A new fine micro-brush (Micro brush international, Grafton, WI
53024, United States) was dipped into one of the agents (NSFGTE
or SDF) and (0.1 ml) was applied to the lesion surface for
one minute. The areas were not rinsed and the tooth surface was
covered with petroleum jelly. Kindergarten teachers were instructed
that participants were not allowed to eat or drink for an hour
after the application (18). Examinations and intervention applications
were carried out in one of the kindergarten classrooms and
applied by the same investigator (AZ).
Clinical evaluation of the lesions was carried out using a visual
and tactile assessment, which was performed 21 days, 3 and 6
months after treatment using (ICDAS II) criteria to assess carious
lesions activity. The assessment was achieved by two blinded calibrated
investigators using a blunt probe and artificial light. Shiny
lesions that felt hard on gentle probing were considered arrested
cavities according to ICDAS II criteria. 10% of the total sample
was randomly re-evaluated on different occasions to monitor
intra-examiner reproducibility.
Participants, parents and biostatistician were blinded toward
agents used in the study by using two identical bottles with tag
codes A and B. Even though outcome examiners were not aware
of which agent was used on the treated tooth, the blinding process
was not guaranteed since the lesions treated with SDF were
dark stained.
Since children with multiple carious lesions were enrolled, each
tooth was treated as a single unit. The Kolmogorov–Smirnov test
was used to analyze the normality distribution of quantitative
data. Intragroup comparisons were made using the Chi-square test with a 95% confidence interval and a two-sample t-test was
used for means comparisons.
For the non-inferiority of caries arrest, the null hypothesis was
that (NSF-GTE) is inferior to the (SDF) by at least 15%. Thus,
the per-teeth percentage of active lesions at baseline treated
with NSF-GTE versus SDF and stayed arrested throughout the
6-months follow-up was analyzed. The non-inferiority margin was
set at 15% according to previous studies [18, 19]. Confidence intervals
were calculated for the difference between the two groups,
with the width of this interval representing the margins of noninferiority.
Even though this is the preferred method to report
non-inferiority results according to the United States for Food
and Drug Administration (FDA) and Consort statement [13, 20],
But P-values were also reported for the rate of caries arrest between
the two groups.
The Co-variables that could possibly alter the treatment effects
on the arrest rate were assessed using a multi-level non-linear logistic
regression model, which was built using Generalized Equation
Estimation (GEE). The dependent variable was caries arrest,
and Co-varieties were (Treatment group, age, gender, deft index,
type of lesion, and site of the lesion). The level of significant
difference was set at 0.05 for all statistical tests. Statistics were
calculated using the (SPSS) software v25.0. Intra-rater reliability
and inter-examiner agreement for caries assessment were calculated
by Cohen’s kappa test. The kappa for intra-rater reliability
was 0.85 and 0.90. The kappa for the inter-examiner agreement
was 0.87.
Results
Sixty-three preschool children (22 males and 39 females) were enrolled
in this study with a mean age of 3.9 and 4.1 years for group
A and B respectively. The mean (deft) index at baseline was (4.1 ±
1.8) for group A and (5.1 ± 0.5) for group B with no statistically
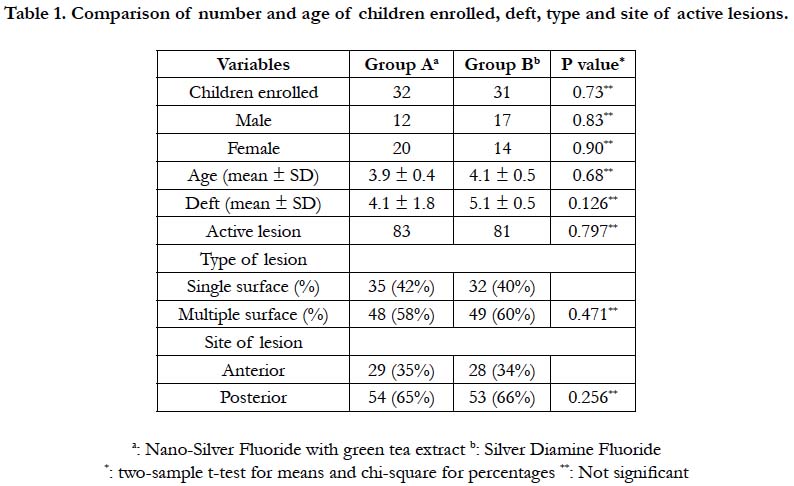
significant difference between the two groups (P> 0.05). (Table 1)
A total of 63 preschoolers with 164 cavitated active lesions were
randomly allocated into two groups: 32 children with 83 lesions
for the Nano-Silver Fluoride with green tea extract group (Group
A) and 31 children with 81 lesions for the Silver Diamine Fluoride
group (Group B).
The number, type, and site of lesions in each group were not identical because some participants had more than one lesion included in this trial, and the protocol required that every participant receive only one type of the agents to avoid the crossover effect. Nevertheless, there were no statistically significant differences between the groups (P> 0.05). (Table 1)
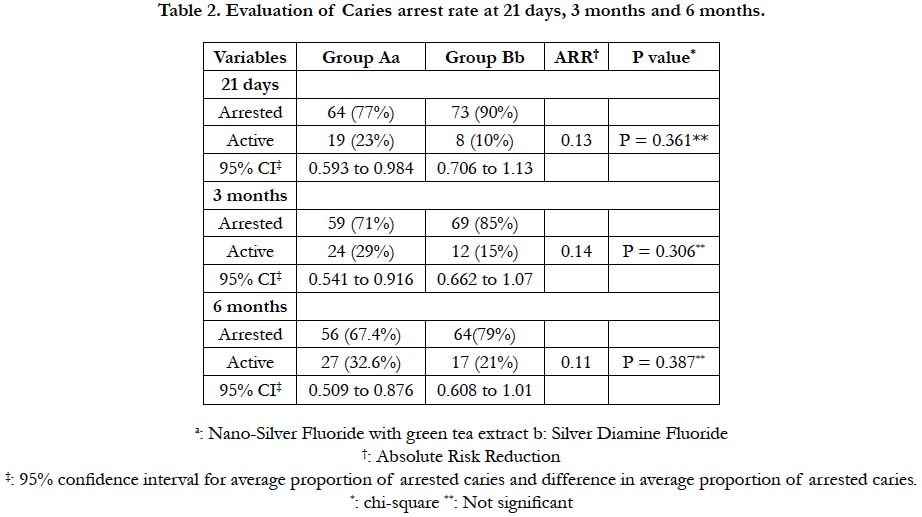
After 21 days, 77% (95% CI: 0.59 to 0.98) of active lesions in group A showed hard arrested dentin, compared with 90% (95% CI: 0.706 to 1.13) of arrested lesions in the group B (P> 0.05). After three months, Group A and B had 71% (95% CI: 0.54 to 0.91) and 85% (95% CI: 0.66 to 1.07) arrest rate respectively (P>0.05). At 6 months, 67.4% (95% CI: 0.50 to 0.87) of active lesions treated with NSF-GTE were still arrested, while 79.6% (95% CI: 0.608 to 1.01) active lesions treated with SDF group remained arrested (95% CI: 0.608 to 1.01) (P>0.05). (Table 2)
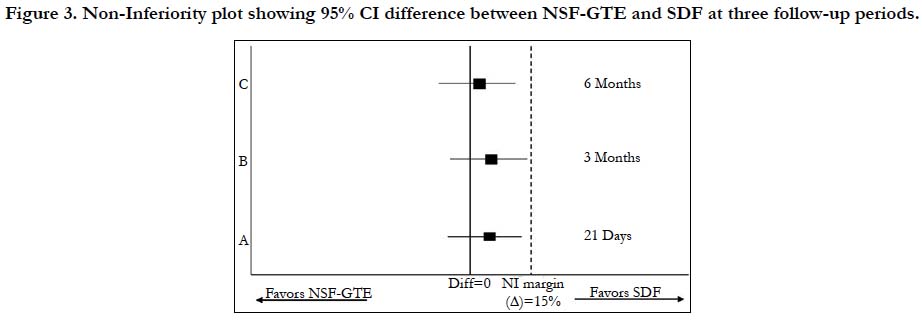
Figure 3 demonstrates the non-inferiority of NSF-GTE as it shows that 95% confidence interval of the Relative Risk for (group A) at the three follow-up periods lies entirely below the predefined margin in comparison to (group B).
Figure 3. Non-Inferiority plot showing 95% CI difference between NSF-GTE and SDF at three follow-up periods.
In the multi-level non-linear logistic regression model, results of age, gender, deft index, and treatment group were not significant. However, the type of lesion and the site of the lesion have affected the caries arrest rate significantly. Regarding the type and size of the lesions, single surface lesions had 1.99 times the chance to become arrested compared to multiple-surface lesions (P< 0.05). Whereas lesions located in anterior teeth had 3.15 times the chance to become arrested compared to posterior lesions (P< 0.05). (Table 3)
Table 3. Multi-level Logistic regression model of the caries arrest rate after 6 months with clustering effect.
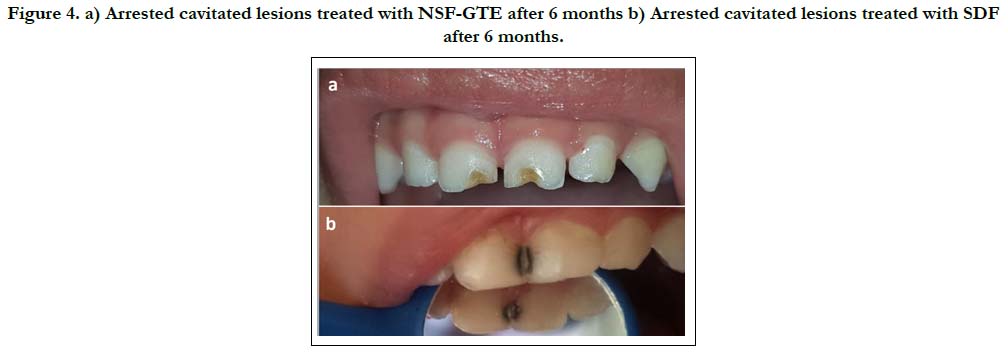
After six months, all lesions treated by SDF (group B) have turned black (Figure. 4a). While lesions of the NSF-GTE (group A) have not in any way turned black. (Figure. 4b)
Figure 4. a) Arrested cavitated lesions treated with NSF-GTE after 6 months b) Arrested cavitated lesions treated with SDF after 6 months.
Discussion
Young children, in impoverished countries with limited access to
health care, suffer from a high incidence of having untreated carious
lesions [21]. Thus, the age group that was chosen to be studied
in this trial was between 3 and 5 years old.
A biological approach using Green tea extract was used in this
study to synthesize AgNps instead of the chemical approach
that was reported by other studies [4, 22]. This new eco-friendly
“green” method of synthesis offers a simple, fast, and a one-step
(biogenic) approach. Studies have shown that green tea extract
can act as a reducing and capping agent for the production of
AgNPs without the need for adding hazardous chemicals or complicated
laboratory devices [8, 9, 23]. On the other hand, chemical
synthesis of nano-Silver particles has raised questionable arguments
since the process contains hazardous substances such as
acetic acid and borohydride sodium [7].
Also, SDF was chosen as an active control since this topical agent
is recently considered the gold standard in arresting dentin caries
in primary teeth [5].
This controlled trial was designed as a non-inferiority trial to test
whether the effect of (NSF-GTE) is not exorbitantly worse than
the effect of SDF 38% by more than a predefined non-inferiority
margin (15%). This design is crucial when the test drug cannot
be compared to a placebo for ethical reasons, especially if this
indicate negating participants an effective drug treatment. Furthermore,
“non-inferiority is used when the test drug is believed
to have slightly worse efficacy compared to the active control but
offers safer, more cost-effective, or better esthetics options treatment
options”[20].
International Caries Detection and Assessment System (ICDAS
II) criteria have been applied in the inclusion and the outcome
assessment in this study. Cavities that had changed from soft
or leathery into shiny and hard were considered arrested caries.
These criteria have been widely studied, and it was shown that
ICDAS II criteria are reliable, valid, and reproducible in assessing
the activity of primary caries [24-28].
In this trial, the first period of follow-up at 21 days after agents’
application is used, to be in accordance with the reported period (21 days) required for Silver Diamine Fluoride to arrest caries [29,
30]. On the other hand, there are no studies about the efficacy of
NSF-GTE nor the time it needs to arrest caries. Therefore 3 and
6 months follow up periods were included to identify the effect of
this agent after more extended periods post-application.
The arrest rate for carious lesion treated with SDF was noticeably
higher than NSF-GTE in all follow-up periods. This increased
rate is likely attributable to the fact that SDF 38% contains much
higher concentrations of Silver Nitrate and Sodium Fluoride than
those in NSF-GTE (5x AgNO3 %, 4x NaF %) [31]. However, the
difference in the arrest rate was not statistically significant, and
this trial demonstrated the non-inferiority of NSF-GTE compared
to SDF.
This trial is the first “in-vivo study” to assess the effectiveness of
a novel Nano-Silver Fluoride with green tea extract (NSF-GTE)
to arrest cavitated lesions. The present study shows that NSFGTE
was effective in arresting dentin carious lesions in both anterior
and posterior primary teeth with the total arrest rate after
six months (67.4%).
A valuable component of green tea is the polyphenols. Polyphenols
possess an antibacterial property that is attributable to the
active ingredient, Catechins. The anti-cariogenic effect of green
tea extract includes many activities [32]. Green tea can prevent
the adhesion of bacteria on the tooth surface [33]. Furthermore,
green tea catechins own a suppressing effect on the development
of S. mutans, which in turn can decrease glucosyltransferase activity
and reduce capsule synthesis [34, 35]. Also, catechins can
reduce plaque acidity and maintain pH towards neutrality, which is
an unfavorable environment for cariogenic bacteria [36].
Silver Nanoparticles (AgNPs) are being widely used for its broadspectrum
antibacterial property. Two approaches can explain the
possible mechanisms of action of Nano-Silver particles. The first
one is the interaction of the AgNPs with the bacterial cell membrane.
AgNPs can establish pits in the cytoplasmic membrane of
the bacteria through reactive oxygen species (ROS), which can
lead to cell death [37].
Second, AgNPs penetrate the membrane followed by a burst
release of Silver ions inside the bacterial cells, which “interacts
with the DNA replications, cell inclusions, enzymes and disrupts
the respiratory chain reactions causing inhibition of cell division,
which may ultimately cause cell death”[38].
In vitro microbial studies showed that AgNPs were effective in
inhibiting S. mutans strains, one of the primary pathogens responsible
for dental caries. AgNPs are both bacteriostatic and
bactericidal [39, 40]. Furthermore, it has been reported that the
antibacterial efficacy of AgNPs increases with smaller particle
size and spherical shape by providing a larger surface area for contact
with bacteria [41]. SEM analysis of the AgNPs in this study
showed spherical AgNPs with approximate sizes of 8 ± 4.4 nm.
To the best of our knowledge, there are only two in vivo studies
in the literature reporting the efficacy of Chemically synthesized
Nano-Silver Fluoride in arresting dentin carious lesions in
primary teeth. Dos Santos et al. reported an arrest rate of 66.7%
by using chemically synthesized Nano Silver Fluoride preparation,
which is equivalent to results obtained in this study (67.4% arrest
rate) [4]. To the contrary, Tirupathi et al. showed a higher arrest rate in Nano-Silver incorporated Sodium Fluoride (NSSF) group
(77%) [22]. This increased rate of arrested lesions could be attributed
to two factors. First, the participating children in Tirupathi
trial were older living in an optimal fluoridate community. Second
factor, Silver nitrate concentration was higher (5%) which could
have played huge role in the arrest rate difference.
The Silver Diamine Fluoride arrest rate in this study (79.6%), is
consistent with other trials reports [18, 22]. However, other studies
have reported an average arrest rate as low as 43% after six
months of treating carious lesions with SDF [19, 42]. This could
be attributed to our study application protocol, which consisted
of applying topical agents for at least one minute and then sealing
cavity with petroleum gel so the agent could stay in contact with
the lesion as long as possible. While in Zhi and Duangthip trials,
SDF was applied for only 10s, and then it was washed away from
the cavity [19, 42].
In this study, primary anterior and posterior teeth with single or
multiple surface lesions were included. It was observed that anterior
teeth and single surface lesions showed higher arrest rates in
comparison to posterior teeth and multiple surface lesions. This
result was statistically significant and can be justified by the fact
that molars and multiple surface lesions have a higher propensity
for plaque accumulation and food impaction. Even more, this result
stems from the fact that incisors and single surface lesions are
easier to clean by the parents for this age group.
In vitro studies have shown the rationality that stands behind the
safety of using NSF-GTE in this study. The use of green tea
polyphenols to synthesize AgNPs is accountable for reducing Ag-
NPs that serve as an effective antioxidant and anti-inflammatory
agents, reducing the possible toxicity of the nanoparticles [8].
In the same context, in-vitro study showed that Silver nanoparticles
synthesized from green tea extract at concentrations up to 100
μg/mL did not exhibit cytotoxicity to HaCaT cells and did not
induce changes in cellular morphology [43]. Another study reported
higher IC50 values for biosynthesized AgNPs against normal
human dermal fibroblasts, which indicates their lower toxicity
in normal cell lines [44]. Targino et al., evaluated the cytotoxicity
of different concentrations of Nano-Silver solutions that ranged
from (1% to 5%), and they found no toxic effect for erythrocyte
[39].
The limitations of this study included a relatively short follow-up
period of six months for the assessment of caries arrest. additionally,
the investigator could not be blinded during the application
process as a result of the different odor and color of both materials.
Furthermore, confounding variables like dietary control and
oral hygiene could not be monitored in the participating children.
It is worth noting that after finalizing the trial, patients with the
remaining active carious lesion in both groups were referred for
treatment in the pediatric dentistry department.
Conclusions
Taken together, this trial demonstrated that Nano-Silver Fluoride
with green tea extract (NSF-GTE) was non-inferior to Silver
Diamine Fluoride (SDF) in arresting cavitated active carious lesions
in primary teeth in preschool children in a non-fluoridated community. NSF-GTE can be considered as an option to treat
decayed primary teeth in underprivileged communities since it is
simple, cost-effective for mass production, and does not necessitate
sensitive application technique.
Further studies with different concentrations and different interval
application with a larger sample size are required to assess the
best protocol for Nano-Silver Fluoride with green tea extract application
are needed.
Author Contributions
Zuhair Al-Nerabieah Contributed to conception, design, data
acquisition, and interpretation, drafted and critically revised the
manuscript; Ettihad Abo Arrag conception, design, data interpretation,
drafted and critically revised the manuscript; Anas Rajab
contributed to design, *Title Page with Author Details data interpretation
and critically revised the manuscript; John C Comisi
contributed, edited and critically revised the manuscript All authors
have read and agreed to the published version of the manuscript.”
Funding
“This research was funded by Damascus University).
Acknowledgments
The authors are grateful to the faculty of pharmacy at Syrian
Private University (SPU) for the experimental support. Special
thanks for Muaz AlKhouli and Salma Al Nesser for serving as
examiners in the trial. We thank Safaa Shehabi for her assistance
with the SEM measurements.
References
- Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–59. PMID: 28919117.
- Organisation for Economic Co-operation and Development. Health at a Glance 2011: OECD Indicators. OECD/Korea Policy Centre, Korea; 2012.
- Chaffee BW, Rodrigues PH, Kramer PF, Vítolo MR, Feldens CA. Oral health‐related quality‐of‐life scores differ by socioeconomic status and caries experience. Community Dent Oral Epidemiol 2017;45:216–24.
- Dos Santos VE, Filho AV, Ribeiro Targino AG, Pelagio Flores MA, Galembeck A, Caldas AF, et al. A new “silver-Bullet” to treat caries in children - Nano Silver Fluoride: A randomised clinical trial. J Dent 2014;42:945–51.
- Crystal YO, Niederman R. Evidence-Based Dentistry Update on Silver Diamine Fluoride. Dent Clin North Am 2019;63: 45–68. PMID: 30447792.
- Hernández-Sierra JF, Ruiz F, Cruz Pena DC, Martínez-Gutiérrez F, Martínez AE, de Jesús Pozos Guillén A, et al. The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomedicine Nanotechnology. 2008;4: 237-40. PMID: 18565800.
- Burns J, Hollands K. Nano Silver Fluoride for preventing caries. Evid Based Dent. 2015;16:8–9. PMID: 25909929.
- Rolim WR, Pelegrino MT, de Araújo Lima B, Ferraz LS, Costa FN, Bernardes JS, et al. Green tea extract mediated biogenic synthesis of silver nanoparticles: Characterization, cytotoxicity evaluation and antibacterial activity. Appl Surf Sci. 2019;463:66–74.
- Kumar V, Wadhwa R, Kumar N, Maurya PK. A comparative study of chemically synthesized and Camellia sinensis leaf extract-mediated silver nanoparticles. 3 Biotech. 2019;9:7.
- Ruttkay-Nedecky B, Skalickova S, Kepinska M, Cihalova K, Docekalova M, Stankova M, et al. Development of New Silver Nanoparticles Suitable for Materials with Antimicrobial Properties. J Nanosci Nanotechnol 2019;19:2762–9.
- Lorenzo JM, Munekata PES. Phenolic compounds of green tea: Health benefits and technological application in food. Asian Pac J Trop Biomed. 2016;6:709–19.
- Elbossaty WF. Green Tea as Biological System for the Synthesis of Silver Nanoparticles. J Biotechnol Biomater 2017;7:2.
- Piaggio G, Elbourne DR, Pocock SJ, Evans SJW, Altman DG, CONSORT Group for the. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. Jama. 2012;308:2594– 604.
- Beiruti N, Taifour M. Prevalence of nursing caries among children 3-5 years old in Damascus. East Mediterr Heal J. 2000;6:500–6. PMID: 11556044.
- Bakhtiari S, Sehatpour M, Mortazavi H, Bakhshi M. Orofacial manifestations of adverse drug reactions: A review study. Clujul Med. 2018;91:27. PMID: 29440948.
- Gugnani N, Pandit IK, Srivastava N, Gupta M, Sharma M. International caries detection and assessment system (ICDAS): A new concept. Int J Clin Pediatr Dent. 2011;4:93. PMID: 27672245.
- ATSDR A for TS and DR. Toxicological profile for silver. Toxicol Profile Silver, US Dep Heal Hum Serv Public Heal Serv Atlanta, GA, 1990. (accessed July 27, 2019).
- Milgrom P, Horst JA, Ludwig S, Rothen M, Chaffee BW, Lyalina S, et al. Topical silver diamine fluoride for dental caries arrest in preschool children: A randomized controlled trial and microbiological analysis of caries associated microbes and resistance gene expression. J Dent. 2018;68:72–8. PMID: 28866468.
- Zhi QH, Lo ECM, Lin HC. Randomized clinical trial on effectiveness of silver diamine fluoride and glass ionomer in arresting dentine caries in preschool children. J Dent. 2012;40:962–7. PMID: 22892463.
- Althunian TA, de Boer A, Groenwold RHH, Klungel OH. Defining the noninferiority margin and analysing noninferiority: An overview. Br J Clin Pharmacol. 2017;83:1636–42. PMID: 28252213.
- Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJL, Marcenes W. Global burden of untreated caries: a systematic review and metaregression. J Dent Res. 2015;94:650–8.PMID: 25740856.
- Tirupathi S, Nirmala SVSG, Rajasekhar S, Nuvvula S. Comparative cariostatic efficacy of a novel Nano-silver fluoride varnish with 38% silver diamine fluoride varnish a double-blind randomized clinical trial. J Clin Exp Dent. 2019;11: e105–12. PMID: 30805113.
- Loo YY, Chieng BW, Nishibuchi M, Radu S. Synthesis of silver nanoparticles by using tea leaf extract from Camellia sinensis. Int J Nanomedicine. 2012;7:4263-7. PMID: 22904632.
- Shoaib L, Deery CM, Ricketts DNJ, Nugent ZJ. Validity and reproducibility of ICDAS II in primary teeth. Caries Res. 2009;43:442–8.
- Braga MM, Ekstrand KR, Martignon S, Imparato JCP, Ricketts DNJ, Mendes FM. Clinical performance of two visual scoring systems in detecting and assessing activity status of occlusal caries in primary teeth. Caries Res. 2010;44:300–8. PMID: 20530964.
- Ekstrand KR, Luna LE, Promisiero L, Cortes A, Cuevas S, Reyes JF, et al. The reliability and accuracy of two methods for proximal caries detection and depth on directly visible proximal surfaces: an in vitro study. Caries Res. 2011;45:93–9. PMID: 21412000.
- Tikhonova SM, Feine JS, Pustavoitava NN, Allison PJ. Reproducibility and diagnostic outcomes of two visual-tactile criteria used by dentists to assess caries lesion activity: a cross-over study. Caries Res. 2014;48:126–36. PMID: 24335157.
- Dikmen B. Icdas II criteria (international caries detection and assessment system). J Istanbul Univ Fac Dent. 2015;49:63-72. PMID: 28955548.
- Liu BY, Lo ECM, Li CMT. Effect of silver and fluoride ions on enamel demineralization: a quantitative study using micro‐computed tomography. Aust Dent J. 2012;57:65–70. PMID: 22369560.
- Zhi QH, Lo ECM, Kwok ACY. An in vitro study of silver and fluoride ions on remineralization of demineralized enamel and dentine. Aust Dent J. 2013;58:50–6. PMID: 23441792.
- Crystal YO, Niederman R. Silver diamine fluoride treatment considerations in children’s caries management. Pediatr Dent. 2016;38:466–71. PMID: 28281949.
- Hamilton-Miller JMT. Anti-cariogenic properties of tea (Camellia sinensis). J Med Microbiol. 2001;50:299–302.
- Ferrazzano GF, Roberto L, Amato I, Cantile T, Sangianantoni G, Ingenito A. Antimicrobial properties of green tea extract against cariogenic microflora: an in vivo study. J Med Food. 2011;14:907–11.
- Subramaniam P, Eswara U, Reddy KRM. Effect of different types of tea on Streptococcus mutans: An in vitro study. Indian J Dent Res 2012;23:43.
- Tao D-Y, Shu C-B, Lo ECM, Lu H-X, Feng X-P. A randomized trial on the inhibitory effect of chewing gum containing tea polyphenol on caries. J Clin Pediatr Dent. 2013;38:67–70.
- Ahmed SI, Sudhir KM, Reddy VCS, Kumar RVSK, Srinivasulu G. Green Tea in the Prevention of Dental Caries A Systematic Review. Int Arch Biomed Clin Res 2017;3:1–6.
- Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346-53. PMID: 20818017.
- Arya G, Sharma N, Mankamna R, Nimesh S. Antimicrobial Silver Nanoparticles: Future of Nanomaterials. Microb. Nanobionics, Springer. 2019; 89-119.
- Targino AGR, Flores MAP, dos Santos Junior VE, Bezerra F de GB, de Luna Freire H, Galembeck A, et al. An innovative approach to treating dental decay in children. A new anti-caries agent. J Mater Sci Mater Med. 2014;25:2041–7. PMID: 24818873.
- Hernández-Sierra JF, Salas-López EK, Martínez-Gutiérrez F, Ruíz F, Pierdant-Pérez M, Mandeville P, et al., Bactericidal capacity of silver nanoparticles associated with Gantrez S-97 on Streptococcus mutans. J Clin Pediatr Dent. 2010;35:183–5. PMID: 21417121.
- Agnihotri S, Mukherji S, Mukherji S. Size-controlled silver nanoparticles synthesized over the range 5-100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014;4:3974-83.
- Duangthip D, Chu CH, Lo ECM. A randomized clinical trial on arresting dentine caries in preschool children by topical fluorides-18 month results. J Dent. 2016;44:57-63. PMID: 26037274.
- Moulton MC, Braydich-Stolle LK, Nadagouda MN, Kunzelman S, Hussain SM, Varma RS. Synthesis, characterization and biocompatibility of “green” synthesized silver nanoparticles using tea polyphenols. Nanoscale. 2010;2:763–70. PMID: 20648322.
- Selvan DA, Mahendiran D, Kumar RS, Rahiman AK. Garlic, green tea and turmeric extracts-mediated green synthesis of silver nanoparticles: Phytochemical, antioxidant and in vitro cytotoxicity studies. J Photochem Photobiol B Biol. 2018;180:243–52.