Evaluation of the Effect of Near Infra-red Photobiomodulation on Buccal Fat Pad-Derived Stem Cells
Leila Gholami1,2, Saeed Afshar3, Roghayeh Mahmoudi3, Ali Asghar Arkian4, Gilda Parsamanesh5, Maryam Rezai Rad2*, Kaveh Baghaei5*
1 Department of Periodontics, Dental implant Research Center, School of Dentistry, Hamadan University of Medical Sciences, Hamadan, Iran.
2 Research Institute for Dental Sciences, Dental Research Center, School of Dentistry, ShahidBeheshti University of Medical Sciences, Tehran, Iran.
3 Research Center for Molecular Medicine, Hamadan University of Medical Sciences,Hamadan, Iran.
4 Dental Research Center, School of Dentistry, Hamadan University of Medical Sciences, Hamadan, Iran.
5 Basic and Molecular Epidemiology of Gastrointestinal Disorders Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
*Corresponding Author
Maryam Rezai Rad, DDS, PhD,
Research Institute for Dental Sciences, Dental Research Center, School of Dentistry, Shahid Beheshti University of Medical Sciences, Evin, Tehran, Iran.
Tel: +989125383022
E-mail: m.rezai.rad@gmail.com
Kaveh Baghaei, PhD,
Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Velenjak, Tehran, Iran.
Tel: +98 0912 359 2868
E-mail: kavehbaghai@gmail.com
Received: October 24, 2020; Accepted: December 01, 2020; Published: December 10, 2020
Citation:Leila Gholami, Saeed Afshar, Roghayeh Mahmoudi, Ali Asghar Arkian, Gilda Parsamanesh, Maryam Rezai Rad, Kaveh Baghaei. Evaluation of the Effect of Near Infra-red Photobiomodulation on Buccal Fat Pad-Derived Stem Cells. Int J Dentistry Oral Sci. 2020;7(12):1164-1171. doi: dx.doi.org/10.19070/2377-8075-20000231
Copyright: Maryam Rezai Rad, Kaveh Baghaei©2020. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Laser photobiomodulation can be a useful adjunctive method in tissue engineering in enhancement of proliferation
and differentiation of mesenchymal stem cells. Buccal fat pad-derived stem cells (BFPSCs)has been introduced as a promising
source for craniofacial bone tissue engineering. Current study aimed to evaluate the effects of near infra-red photobiomodulation
on (BFPSCs)behavior.
Methods: After cell isolation from a surgically excised sample of human buccal fat pad, third passage cells were irradiated twice
daily for three consecutive days. Irradiation was performed with 6 different laser settings by two modes of continuous and pulsed
(50% duty cycle) and energy densities of 3 and 6 J/cm2 and two different output powers (0.1W and 0.3W) using a 940nm laser.
Anon-irradiated group served as control. The test was repeated in three different daysand every time cell viability was evaluated by
MTT assay at intervals of 24, 48 and 72h. Based on viability results a setting was chosen for evaluation of osteogenic differentiation
by Alizarin red staining.
Results: The highest proliferation was observed at irradiation of 3J, 0.3W, pulsed at 24h and 48h, however, after 72h the highest
proliferation rate was related to 6J ,0.1W, pulsed. Considering the effect of 3J 0.3W pulsed modeon cell proliferation at an earlier
time, this setting was used for osteogenic differentiation assay. Both microscopic and quantitative analysis of Alizarin Red staining
showed that cells subjected to the 3J 0.3W Pulsed irradiation also resulted in an increase in mineralization of BFPSCs cultured in
osteogenic induction medium compared to the negative control (p<0.05).
Conclusion: According to the results of this study a pulsed mode of irradiations showed better viability results. Although the 3J/
cm2 0.3W, Pulsed irradiation showed significantly better results for viability and proliferation, however no statistically significant
effect was observed in osteogenic differentiation.
2.Introduction
3.Methods
4.Results
5.Discussion
6.Conclusion
7.Acknowledgments
8.Refereces
Keywords
Buccal Fat Pad-Derived Stem Cells; Photobiomodulation: Cell Proliferation: Cell Differentiation.
Introduction
Evidence of the use of Photobiomodulation (PBM) by humans
goes back to thousands of years ago in ancient civilizations where
they used sunlight sometimes combined with plants for treatment
of skin diseases [1]. Years later the Nobel Prize of Physiology and
Medicine was awarded to Nils Finsen for his invention in using
arc lamps to treat cutaneous tuberculosis and prevention of scarring
from smallpox [2, 3]. Low level laser (light) therapy (LLLT) or
more recently regarded as photobiomodulation is a nonthermal
process that its biostimulatory effect was accidently discovered by
Endre Mester in 1960 [4, 5]. Ever since various wavelengths of laser or LED light have been tested for their photobiomodulatory
effects in many in vitro and in vivo studies.
Previous studies on the biomodulatory effects of Laser therapyhave
reported positive effects on cell proliferation, tissue regeneration
and anti-inflammatory potentials [6-9]. However, due to
inconsistencies in laser settings, cell types and treatment protocols
and lack of well controlled clinical trials of photobiomodulation
in different fields it has not yet become practical in the medical
field and a clear protocol or guideline does not exist [6, 9, 10].
A biphasic dose response has been observed in many reports described
as Arndt-Schulz curve showing that only irradiation doses
within a specific range may have biostimulatory results and very
low or very high doses may even lead to inhibitory or negative results
[11]. Irradiation factors other than energy density or fluence
(J/cm2) which influencing the results of photobiomodulation. include
power density, irradiation durations and number of applications
and also, the continuous or pulsed mode of emission. The
mechanisms behind PBM and the results observed on tissue and
cell has also been an interesting topic of many researches [5, 12]
Some accepted biological mechanism of PBM include the primaryabsorption
by cellular chromophores such as enzymes likemitochondrial
cytochrome c oxidase, porphyrin and flavoproteins
and membrane photoreceptors. Secondary effects of this photon
absorption may result in increases in ATP, reactive oxygen species
(ROS), increase in nitric oxide, and modulation of calcium levels.
Tertiary effects include activation of transcription factors resulting
in changes in cell survival, proliferation and migration, and
new protein synthesis [10, 13, 14].
Tissue engineering has revolutionized oral and maxillofacial and
periodontal regenerative therapies and many different stem cell
sources such as cells with craniofacial and dental origins seem to
be promising for this novel field of cell therapy treatments [15-
17]. Methods that are capable of increasing cell survival and proliferation
and differentiation used as adjunctive either in ex-vivo
expansion of cells or in-vivo on the treated area is of great value
for regenerative medicine. PBM is a suitable adjunctive tool for
this purpose. Despite the great number of researches on the effect
of PBM on cell such as mesenchymal stem cells (MSC) used
in tissue engineering there are still no clear optimal parameters
and irradiation protocolsdefined. These effects seem to depend
on the cell type and the irradiation settings [6, 7].
In search for ideal and easily accessible sources of stem cells for
craniofacial tissue engineering Farre-Guasch et al., isolated adipose-
derived stem cells (AdSCs) from Bichat’s fat pad or the buccal
fat pad (BFP). This is a highly vascularlized fat mass which
has been an attractive graft, in oral surgery for the repair of bone
and periodontal defects. It is located on both sides of the face between
the buccinator muscle and other superficial muscles and is
easily accessible through the oral cavity with minimal discomfort
and donor site morbidity [18]. These cells are phenotypically similar
to AdSC from abdominal subcutaneous adipose tissuein cell
yield, morphology, and multilineage differentiation [18-20]. They
have also been reported to proliferate faster and is more prone to
producing colonies compared to other AdSCs. These cells were
demonstrated to be capable of reliably forming engineered bone
in an invivo study by Shiraishi et al., [21]. The clinical application
of these cells in bone regeneration has also been positively reported
in some studies [22-24]. Regarding the effect of PBM on adipose derived stem cells(BFPSCs), there are a few investigating
the effects of phototherapy on proliferation and differentiation
of these cells with varying light wavelengths and irradiation parameters
[25-30]. The combination of laser photomodulation and
adipose stem cells has been also studied for many different clinical
applications with successful positive outcomes [31, 32]. Showing
a promising potential for their applicability. However, up to our
knowledge the effect of PBM of BFP -ASChas not been investigated
previously and the effect of different pulsed and continuous
irradiation settings of the near infra-red (NIR) wave length
has not been investigated previously.
The near infra-red laser was chosen in this study since it has a
more deeper penetration depth compared to red lasers making it
a suitable choice for future translation of this technique to clinical
practice in craniofacial bone tissue engineering. However, determination
ofideal irradiation parameters is important to the standardization
of a PBMfor achieving favorable results on proliferation
and differentiation of cells.
Therefore,In the present study we aimed to comparatively evaluated
the effect of different irradiation parameters ofpulsed and
continuous 940nm near infra-red diode laser PBM on proliferation
and osteogenic differentiation of Buccal fat pad derived Adipose
stem cells.
BFP tissues were collected froma healthy individual who needed
maxillofacial surgery after obtaining an informed consent. The
isolated tissue (approximatly 10mm 3) was transferred to the cell
culture laboratory in chilled phosphate buffer solution (PBS)
(Life Technologies, Carlsbad, CA, United States). Then, tissues
were minced and digested in 3 mg/mL collagenase type I (Life
Technologies, Carlsbad, CA, United States) for one hour at 37 °C.
The suspended cells, were cultured in Dulbecco's Modified Eagle
Medium (DMEM, GIBCO BRL, Grand Island, NY), 15% fetal
bovine serum (FBS) (Life Technologies, Carlsbad, CA, United
States), and 1% Penicillin-Streptomycin 10,000 u/ml (Life Technologies,
Carlsbad, CA, United States). Primary cells were passed
upon confluency using 0.25% trypsin-EDTA (Life Technologies.
Carlsbad, CA, United States). Cells at passage two were characterized
for mesenchymal stem cell surface markers. Briefly, cells were
trypsinized andthen, they were incubated in darkness for one hour
at 4 °C with specific antibodies of CD90, CD73, CD105 markers
as mesechymal stem cell markers and CD34, CD45 markers as a
hematopoietic cell marker. (EXBIO Praha,Vestec, Czech Republic)
at 2 μg/ml for each. Finally, expression of these molecules
were analyzed by FACSCalibur Flow cytometer (Becton Dickinson,
San Jose, CA), and the data were analyzed using FlowJo (Tree
Star, Ashland, OR) software.
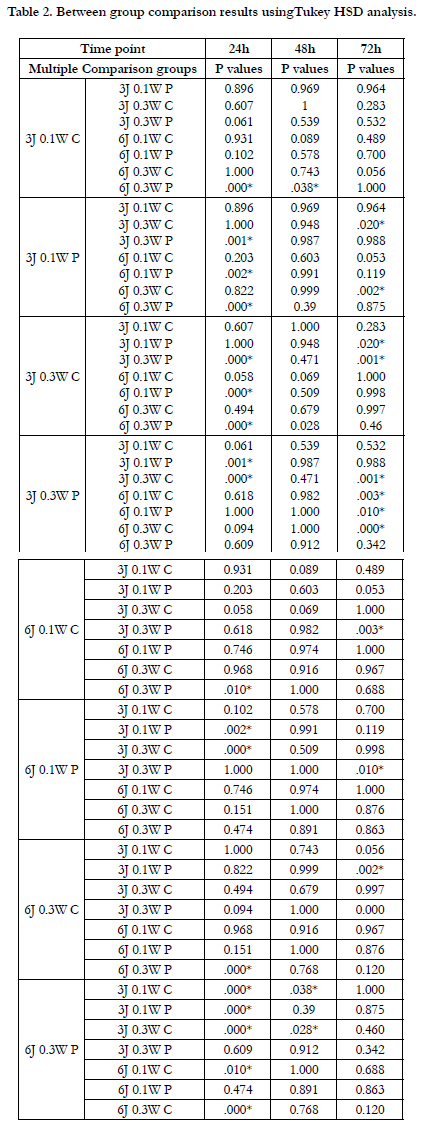
BFPSCs were seeded in 96-well plates at a density of 2 × l03 per
well and cultured in DMEM, 15% FBS, 1% Penicillin-Streptomycin.
Then, the next day, cells were subjected to irradiation according
to Table 1.
A 940nm InGaAsP Semi-conductor diode laser (Biolase, USA) was used in continuous and pulsed mode of irradiation with two energy densities of 3 and 6 J/cm2 and out put powers of 0.1 or 0.3W. Cells were irradiated from underneath the wells by fixing the laser handpiece in a position perpendicular to the bottom of the plates in a distance to create a spot size equal to the diameter of a single well. The transmitted output power through the transparent bottom of plates was measured using a power meter (Nova II, Ophir photonics) to be sure of the correct power reaching the cells from the base of the plates. Cells were seeded every other well and wells not being irradiated were covered by dark cardboard during laser irradiation to avoid unintentional dispersion of light between the wells. The control groups were processed under the same conditions, except without laser irradiation. Irradiation was performed every 12h for three consecutive sessions. For each group 6 wells were considered and the test was repeated three times (n=18) for better reliability and reproducibility of the results.
The best setting was chosen for evaluation of its effect on osteogenic differentiation of cells for this test cells were cultured in 6 well plates and a therapy handpiece was used to irradiate each well from underneath. This hand piece was also fixed perpendicular to the plates at a distance(1cm) creating a spot size equal to the size of a single well of a 6 well plate (9.6cm2). The cells were seeded in a osteogenic induction medium irradiated with 3J/cm2 0.3W pulsed mode for 96sec every 12h for three consecutive days. (OIM-3). A group with no irradiation served as a positive control (OIM-0) and we also cultured cells in non-osteogenic medium without any laser irradiation as a negative control (C_).
Immediately after irradiation, cells were returned to incubator
providing 50% CO2 and 37°C. After 24, 48, and 72h, cell viability/
proliferation were evaluated using 3-(4,5-dimethylthiazol-
2-yl)-2, 5-diphenyltetrazolium bromide (MTT) solution (Sigma,
St. Louis, Missouri, USA) (5g/L). Briefly, 10 μl of MTT solution
(5 mg/ml) dissolved in 90 μl of medium was added to each well
and the plates were incubated for 2 h at 37°C. The absorbance
was measured at 570 nm by ELIZA reader (BioTek, Winooski,
VT, USA). The best irradiation setting based on viability results were used for following osteogenic differentiation test.
BFPSCs were seeded in 6-well plates at a density of 5 × l03 per
well and cultured in DMEM, 15% FBS, 1% Penicillin-Streptomycin
for 48h. Then, cells were irradiated with the 3J/cm2 0.3W
Pulsed for 96 sec and incubated in osteogenic medium containing
DMEM, 10% FBS, 100 nM dexamethasone, 0.2 mM ascorbic
acid, and 10 mM β-glycerophosphate (Sigma, St. Louis, Missouri,
USA). After 14 and 21 days, the capability of cells osteogenic
differentiation were measured using Alizarin Red staining (Sigma,
St. Louis, MO, USA), which stains the precipitated calcium in the
matrix.
Stained cells were imaged using optical microscopy. For quantitative
analysis, cell layer was covered by mixing 15% acetic acid and
20% methanol for 45min.Then, the optical density of the solution
was read at 405 nm.
All experiments were conducted in 6 wells for each group repeated
at three different time points. First, the normal distribution of
MTT data was tested using a k-s sample test. three-way analysis
of variance (Three way ANOVA) and Tukey HSD was used for
between group comparisons of the different laser settings. One
sample t-test was also used to significantly compare the rate of
MTT changes in groups compared to the control group. Data
were analyzed by GraphPad Prism software version 8.0.1. Kolmogorov
Smirnov test was used to examine the data normality.
Mean values were compared by independent samples t-test for
data with normal distribution; otherwise, Mann-Whitney U-test
was used. P values of <0.05 (*), <0.01 (**), and <0.001 (***) were
considered significant at different levels.
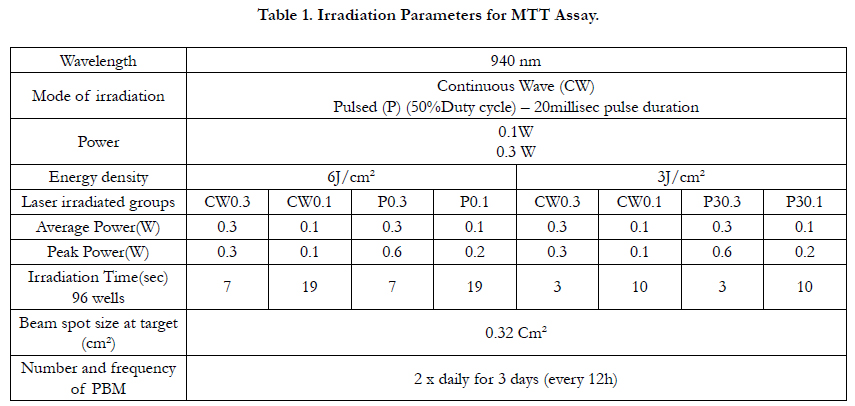
Figure 1 shows the results of MTT assay at different time points.
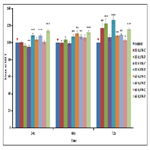
Also, the results of multiple comparison at different time pointscis
presented in Table 2. There was no statistically significant difference between the two different output powers ant any of the
time points, Pulsed mode of irradiation results showed statistically
significant differences with better outcomes for pulsed mode
(P<0.05) In terms of energy density as it shown in Figure 1, the
highest proliferation capability was observed at irradiation of 3J
0.3W P at 24h and 48h, however, after 72h the highest proliferation
rate was related to 6J 0.1W P. Considering the effect of 3J
0.3W P on cell proliferation at an earlier time, irradiation with this
setting was used for osteogenic differentiation assay.
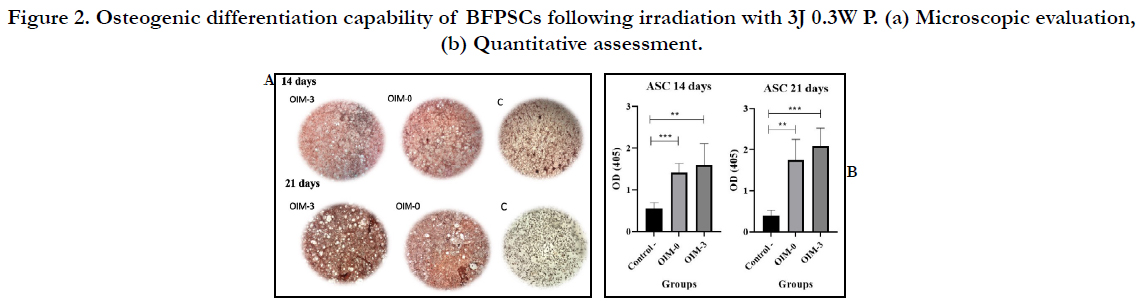
Both microscopic and quantitative analysis (Figure 2 a and b) of
Alizarin Red staining showed that cell subjected to 3J 0.3W P irradiation
had statistically increased mineralization compared to the
negative control group (p<0.05). However, there was no statistically
significant difference between the quantitative evaluation
of mineralized tissue deposition after 14 and 21 days between
the OIM-3 and OIM-0 group with Pvalues of 0.53 and 0.097,
respectively.
Figure 2. Osteogenic differentiation capability of BFPSCs following irradiation with 3J 0.3W P. (a) Microscopic evaluation, (b) Quantitative assessment.
Discussion
Positive effects of PBM on cellular biological behaviors, including
cell proliferation and differentiation of various cell types such
have been reported previously…ref, however, the optimal parameters
for effective bio stimulation of cells needs further well-designed
evaluations.
In the present study we aimed to study the effects of pulsed and
continuous near infra-red laser irradiation with two different energy
densities and output powers on proliferation and osteogenic
differentiation of BFPSCs which is considered as a potentially
suitable stem cell source for craniofacial tissue engineering.
According to the results of the present study a pulsed mode of
irradiation resulted in significantly better outcomes for the proliferation
of BFPSCs at all time points.(p<0.05) however, the different
powers did not have statistically significant differences.
72 h after irradiation the highest MTT results were observed in
the 6J 0.1W Pulsed group, however the 3J 0.3W Puled mode of irradiation
was chosen as the best irradiation setting since it resulted
in significantly better MTT result at all time points of (24,48,72h)
compared to controls and higher viability results at earlier time
points of 24 and 48 h compared to the 6J 0.1W Pulsed group.
Due to the great number of studies on the effect of PBM on stem
cells we mostly have focused on studies which have been conducted
on Adipose stem cells (ASCs) for comparisons. Up to our
knowledge there is no study on the PBMof BFPSCs until now.
However, the effect of PBM on ASC has been the topic on some
studies, although many different wavelengths and irradiation parameters
have been employed, which makes precise comparisons
difficult [6, 7].
In a study by Anwer et al., the green 532nm laser light with energy
densities of 5, 6.8, 9.2, 28 and 45 J/cm2 were used and they observed
that high energy densities with longer exposures resulted
in significant decrease in proliferation which is in accordance with
the Arndt-Schulz law [11, 33].
Other studies have mostly studied the effects ofred laser for PBM
[26, 31, 34-36]. However, they have all observed a continuous
mode of irradiation of a red laser is able to improves proliferation
and cell viability of ASC. However, many different laser energy
densities and output powers have been used.
In a recent study by De Andrade et al., PBM with 660nm red
laser with an energy of 0.56 and 1.96 J promoted proliferation of
ASCs, but a higher energy setting of 5.04 J was found to be harmful.
In this study they used a 660-nm laser and power of 40 mW
[28]. This might be due to the fact that the corresponding energy
densities applied for the energies utilized in their study were 20,
70, 180 J/cm2 which were much higher than the energy densities
used in the present report.
Wang et al., comparatively investigated the effect of four different
wavelengths of 420, 540, 660, 810nm on AdSCs. They showed
that blue/green irradiation had inhibitory effects on proliferation
and reduced cellular Adenosine Tri Phosphate (ATP) while
red/NIR stimulated proliferation, all at 3J/cm2 and also increased
ATP in a biphasic manner [37].
Similarly,we have shown thatpositive result with observed with
the 3J/cm2 energy density.
However, none of these studies have evaluated the effect of
pulsed mode of irradiation on ASCsIn a different study by Wang
et al two different wavelengths of the near infra-red spectrum,
810nm and 980nm were comparatively evaluated their effects on
ASCs. Interestingly they reported that although the wavelengths
showed a biphasic dose response, but 810 nm had a peak dose
response at 3 J/cm2 for stimulation of proliferation at 24 h, while
the peak dose for 980 nm was 10-100 times lower at 0.03 or 0.3
J/cm2 [38].
Based on these findings it seems that PBM studies are very complicated
and need detailed study of each wave length of the electromagnetic
spectrum needs to be evaluated individually.In this
study we used a 940nm which is potentially be a more suitable
adjunctive laser wavelength for clinical application in craniofacial
bone regenerative treatments due to its deeper penetration depth
compared to green or red lasers.
According to the finding of the present study the laser with that
wavelength and settings utilized had different effects on the proliferation
and differentiation of BFPASC. While cell proliferation
was significantly increased the biominelalization results did not
show a statistically significant effect for the same laser irradiation.
This difference of effect of PBM settings and differences in
effects of wavelengths on stem cell proliferation and differentiation
has also been previously reported by some researchers [37,
39, 40]. Which could be attributed to the difference in underling
signaling pathways that needs to be further elucidated in future
studies.
There have not been many studies investigating the effect of PBM on osteogenic differentiation of ASCs. In a recent report
on PBM of ASCs by Ates et al., both red 635nm and 809nm
near infra-red lasers were studied on their effect on ASC proliferation
and osteogenic differentiation with three energy densities of
0.5, 1.5 and 2 J/cm2 in continuous mode [25]. According to their
resultscell proliferation was not changed significantly which was
different to our results and might be due to the lower energy densities
used in their studyand the use of a continuous wavelength.
Another difference that might be the reason for this in significant
change might be that they have evaluated MTT levels after 7 and
14 days. However according to their alizarin red staining results
for evaluation of mineralization at day 14 the 809 nm irradiation
at all energy densities increased mineralization and in the 2 J/
cm2 group of 635 nm laser also resulted in significantly increased
results of mineralization based on normalized optical absorbance
results. In the present study we similarly observed biomineralization
of ASC in OIM compared to the control as shown by Alizarin
red staining resultsafter 14 and 21 days. However, our results
did not indicate a statistically significant difference between the
laser irradiated group in OIM and the OIM without laser irradiation
at any of these time points.
Near infra-red wavelength PBM either by laser or LED has been
reportedto have positive effectson proliferation and differentiation
of other types of stem cell [41, 44]. Looking at the results
of studies with similar wavelengths to the one used in the present
study only a few was found.
Paschalidou et al have used a similar 940nm laser device to ours
in order to evaluate the effect of 4,8,16J/cm2 irradiation on viability/
proliferation, migration, odontogenic differentiation, and
biomineralization of stem cells from human exfoliated deciduous
teeth (SHED). Their results were consistent with ours and they
reported an increase in proliferation with overall higher results
for 4 J/cm2 and 16 J/cm2. They also evaluated in vitro biomineralization
potential by alizarin red staining and found significantly
higher mineral deposition in the 8j/cm2 group [41].
Although the results of the present study confirm the results of
previous reports of PMB using near-infra red irradiation. The
majority of previous studies have focused on the effect of energy
densities in PBM with only a continuous mode of irradiation [6,
7].
In the present study, we found no difference between power densities
and both energy densities of 3 and 6 j which are regarded as
within the biostimulatory, range were capable of increasing proliferation.
However interestingly our results revealeda statistically
significant positive effect with the pulsed mode of irradiation and
no statistically significant difference in the continuous mode irradiated
groups was observed compared to controls.
Continuous wave or pulsed modes of irradiation may have different
biological effects. Some reports have also indicated even
better biological effects of Pulsing in Low-Level Light or PBM
Therapy, which is consistant with the findings of the present report
[45]. This might be explained by the fact thatthe pulse-off
times may allow a rest time for the irradiated tissue and also the
higher peak powers produced which might result in the differences
observed. This high peak powers production is while the
total energy is kept the same,which leads to less thermal effects
and deeper penetration [45, 46].
There have been a few in vitro studies of PBM with pulsed mode
of diode lasers ona variety of cell lines such asbone marrow stem
cells, osteoblasts, fibroblasts, normal human neural progenitor
cells, [47-51] While only a few studies have comparatively studied
pulsed and continuous irradiation modes on cells [46, 52, 53].
Kim et al have reported an interesting pulse frequency dependency
of PBM in the differentiation of hDPSCs by applying 810nm
LED and evaluating the effects of different frequencies of pulsed
mode. Ueda et al have demonstrated that low-frequency pulsed
830nm laser irradiation significantly stimulates bone formation
compared to continuous irradiation [53]. Pulsed near infra-red irradiation
has also attracted a lot of attention as shown effective
results as a therapeutictool of in wound healing and neurology
with more beneficial results compared to continuous wave specially
in deep tissue repair [54-58].
It is believed that pulsed PBM can promote light-biological system
interactions.This can be explained with the fact that some
fundamental frequencies in biological systems have some fundamental
frequencies that are in the range of tens to hundreds Hz,
are similar to the pulsing frequencies used in pulsed PBM. This
time period could be for instance the half-life of an ion channel in
the mitochondrial membrane. Another reason for improved biological
effects of pulsed irradiation could be its effect on the cellular
levels of mechanisms of action of PBM for instance pulsed
mode multiple photodissociation of nitric oxide from a protein
binding site may be possible which can prevent its rebound observed
in continuous mode. More research is needed for understanding
the exact mechanisms involved with pulsed irradiations
in PBM [45, 55].
Howeverpulsation frequency, pulse duration, duty cycle, duration
of dark period between pulses, peak and average intensities all
are important parameters when comparing pulsed and continuous
modes of the same wavelengths which need to be considered and
evaluated in future studies [59].
Conclusion
According to the results of this study a pulsed mode of irradiations
showed better viability results. Although the 3J/cm2 0.3W,
Pulsed irradiation showed significantly better results for viability
and proliferation, however no statistically significant effect was
observed in osteogenic differentiation. Further investigations are
needed to optimize the settings of this adjunctive treatment technique
and effectively translate it into clinical application of bone
tissue engineering.
Acknowledgments
This study was supported by Research Institute for Dental Sciences,
Shahid Beheshti University of Medical Sciences.
References
- McDonagh AF. Phototherapy: from ancient Egypt to the new millennium. J Perinatol. 2001 Dec;21 Suppl 1:S7-S12. Pubmed PMID: 11803408.
- Nobelstiftelsen. Physiology Or Medicine. Nobel Foundation; 1967.
- Finsen NR. The Red Light Treatment of Small-Pox. Br Med J. 1895 Dec 7;2(1823):1412-4. Pubmed PMID: 20755859.
- Gáspár L. Professor Endre Mester, the father of photobiomodulation. J Laser Dent. 2009;17(3):146–8. Pubmed PMID: 28783466.
- Chung H, Dai T, Sharma SK, Huang Y-Y, Carroll JD HM. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40(2):516– 33. Pubmed PMID: 22045511.
- Hosseinpour S, Fekrazad R, Arany PR, Ye Q. Molecular impacts of photobiomodulation on bone regeneration: A systematic review. Prog Biophys Mol Biol. 2019 Dec;149:147-159. Pubmed PMID: 31002851.
- Marques MM, Diniz IMA, de Cara SPHM, Pedroni ACF, Abe GL, D’Almeida-Couto RS, et al. Photobiomodulation of dental derived mesenchymal stem cells: a systematic review. Photomed Laser Surg. 2016;34(11):500–8. Pubmed PMID: 27058214.
- Escudero JSB, Perez MGB, de Oliveira Rosso MP, Buchaim DV, Pomini KT, Campos LMG, et al. Photobiomodulation therapy (PBMT) in bone repair: A systematic review. Injury. 2019;50(11):1853–67. Pubmed PMID: 31585673.
- Gholami L, Asefi S, Hooshyarfard A, Sculean A, Romanos GE, Aoki A, et al. Photobiomodulation in periodontology and implant dentistry: Part I. Photobiomodulation, Photomedicine, Laser Surg. 2019;37(12):1–26.PubmedPMID: 31750783.
- Hamblin MR. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017;4(3):337–61.Pubmed PMID: 28748217.
- Hamblin MR, Huang YY, Sharma SK, Carroll J. Biphasic dose response in low level light therapy - an update. Dose-Response. 2011;9(4):602–18. Pubmed PMID: 22461763.
- Hamblin MR, Demidova TN. Mechanisms of low level light therapy. In: Mechanisms for low-light therapy. 2006; 614001.
- Bayat M, Chien S. Combined Adipose-Derived Mesenchymal Stem Cells and Photobiomodulation Could Modulate the Inflammatory Response and Treat Infected Diabetic Foot Ulcers. Photobiomodulation, Photomedicine, Laser Surg. 2020;38(3):135–7. Pubmed PMID: 31638476.
- Romagnoli E, Cafaro A. PBM. Theoretical and applied concepts of adjunctive use of LLLT/PBM within clinical dentistry. In: Lasers in Dentistry-Current Concepts. Springer,Cham. 2017; 131–60.
- Horst O V, Chavez MG, Jheon AH, Desai T, Klein OD. Stem cell and biomaterials research in dental tissue engineering and regeneration. Dent Clin. 2012;56(3):495–520. PubmedPMID: 22835534.
- Xu XY, Li X, Wang J, He XT, Sun HH, Chen FM. Concise Review: Periodontal Tissue Regeneration Using Stem Cells: Strategies and Translational Considerations. Stem Cells Transl Med. 2019;8(4):392-403. Pubmed PMID: 30585445.
- . Ahmad F. Stem Cells: A Gold Mine in Dental Research and Tissue Engineering. Cancer Med J. 2019;2(2):41-4.
- Farré-Guasch E, Martí-Pagès C, Hernández-Alfaro F, Klein-Nulend J, Casals N. Buccal fat pad, an oral access source of human adipose stem cells with potential for osteochondral tissue engineering: An in vitro study. Tissue Eng - Part C Methods. 2010;16(5):1083–94. Pubmed PMID: 20078198.
- Broccaioli E, Niada S, Rasperini G, Ferreira LM, Arrigoni E, Yenagi V, et al. Mesenchymal stem cells from Bichat’s fat pad: In vitro comparison with adipose-derived stem cells from subcutaneous tissue. Biores Open Access. 2013;2(2):107–17. Pubmed PMID: 23593563.
- Niada S, Ferreira LM, Arrigoni E, Addis A, Campagnol M, Broccaioli E, et al. Porcine adipose-derived stem cells from buccal fat pad and subcutaneous adipose tissue for future preclinical studies in oral surgery. Stem Cell Res Ther. 2013;4(6):148. Pubmed PMID: 24330736.
- Shiraishi T, Sumita Y, Wakamastu Y, Nagai K, Asahina I. Formation of engineered bone with adipose stromal cells from buccal fat pad. J Dent Res. 2012;91(6):592–7. Pubmed PMID: 22538411.
- Nagasaki R, Mukudai Y, Yoshizawa Y, Nagasaki M, Shiogama S, Suzuki M, et al. A Combination of Low-Intensity Pulsed Ultrasound and Nanohydroxyapatite Concordantly Enhances Osteogenesis of Adipose-Derived Stem Cells from Buccal Fat Pad. Cell Med. 2015;7(3):123–31.Pubmed PMID: 26858900.
- Khojasteh A, Sadeghi N. Application of buccal fat pad-derived stem cells in combination with autogenous iliac bone graft in the treatment of maxillomandibular atrophy: a preliminary human study. Int J Oral Maxillofac Surg. 2016;45(7):864–71. Pubmed PMID: 26846793.
- Khojasteh A, Kheiri L, Behnia H, Tehranchi A, Nazeman P, Nadjmi N, et al. Lateral Ramus Cortical Bone Plate in Alveolar Cleft Osteoplasty with Concomitant Use of Buccal Fat Pad Derived Cells and Autogenous Bone: Phase I Clinical Trial. Biomed Res Int. 2017;2017:6560234. Pubmed PMID: 29379800.
- Ate\cs GB, Ak A, Garipcan B, Gülsoy M. Photobiomodulation effects on osteogenic differentiation of adipose-derived stem cells. Cytotechnology. 2020 Apr;72(2):247-258. Pubmed PMID: 32016710.
- . de Villiers JA, Houreld NN, Abrahamse H. Influence of Low Intensity Laser Irradiation on Isolated Human Adipose Derived Stem Cells Over 72 Hours and Their Differentiation Potential into Smooth Muscle Cells Using Retinoic Acid. Stem Cell Rev Reports. 2011;7(4):869–82. Pubmed PMID: 21373882.
- Ong WK, Chen HF, Tsai CT, Fu YJ, Wong YS, Yen DJ, et al. The activation of directional stem cell motility by green light-emitting diode irradiation. Biomaterials [Internet]. 2013;34(8):1911–20. Pubmed PMID: 23261211.
- de Andrade ALM, Luna GF, Brassolatti P, Leite MN, Parisi JR, de Oliveira Leal ÂM, et al. Photobiomodulation effect on the proliferation of adipose tissue mesenchymal stem cells. Lasers Med Sci. 2019;34(4):677–83. PubmedPMID: 30284088.
- Ginani F, Soares DM, Rocha HAO, Barboza CAG. Low-level laser irradiation promotes proliferation of cryopreserved adipose-derived stem cells. Einstein (Sao Paulo). 2017 Jul-Sep;15(3):334-338. Pubmed PMID: 29091156.
- Zare F, Moradi A, Fallahnezhad S, Ghoreishi SK, Amini A, Chien S, et al. Photobiomodulation with 630 plus 810 nm wavelengths induce more in vitro cell viability of human adipose stem cells than human bone marrowderived stem cells. J Photochem Photobiol B. 2019 Dec;201:111658. Pubmed PMID: 31710923.
- Choi K, Kang BJ, Kim H, Lee S, Bae S, Kweon OK, et al. Low-level laser therapy promotes the osteogenic potential of adipose-derived mesenchymal stem cells seeded on an acellular dermal matrix. Vol. 101 B, Journal of Biomedical Materials Research - Part B Applied Biomaterials. 2013 Aug;101(6):919-28. Pubmed PMID: 23529895.
- Ebrahimpour-Malekshah R, Amini A, Zare F, Mostafavinia A, Davoody S, Deravi N, et al. Combined therapy of photobiomodulation and adiposederived stem cells synergistically improve healing in an ischemic, infected and delayed healing wound model in rats with type 1 diabetes mellitus. BMJ Open Diabetes Res Care. 2020;8(1)::e001033. Pubmed PMID: 32098898.
- Anwer AG, Gosnell ME, Perinchery SM, Inglis DW, Goldys EM. Visible 532 nm laser irradiation of human adipose tissue-derived stem cells: effect on proliferation rates, mitochondria membrane potential and autofluorescence. Lasers Surg Med. 2012;44(9):769–78. Pubmed PMID: 23047589.
- Mvula B, Mathope T, Moore T, Abrahamse H. The effect of low level laser irradiation on adult human adipose derived stem cells. Lasers Med Sci. 2008;23(3):277–82. Pubmed PMID: 17713825.
- Mvula B, Moore TJ, Abrahamse H. Effect of low-level laser irradiation and epidermal growth factor on adult human adipose-derived stem cells. Lasers Med Sci. 2010;25(1):33. Pubmed PMID: 19172344.
- Kim HK, Kim JH, Abbas AA, Kim D-O, Park S-J, Chung JY, et al. Red light of 647 nm enhances osteogenic differentiation in mesenchymal stem cells. Lasers Med Sci. 2009;24(2):214–22. PubmedPMID: 18386092.
- Wang Y, Huang YY, Wang Y, Lyu P, Hamblin MR. Red (660 nm) or near-infrared (810 nm) photobiomodulation stimulates, while blue (415 nm), green (540 nm) light inhibits proliferation in human adipose-derived stem cells. Sci Rep. 2017 Aug 10;7(1):7781.Pubmed PMID: 28798481.
- Wang Y, Huang YY, Wang Y, Lyu P, Hamblin MR. Photobiomodulation of human adipose-derived stem cells using 810nm and 980nm lasers operates via different mechanisms of action. Biochim Biophys Acta Gen Subj. 2017 Feb;1861(2):441-449. Pubmed PMID: 27751953.
- Tani A, Chellini F, Giannelli M, Nosi D, Zecchi-Orlandini S, Sassoli C. Red (635 nm), near-infrared (808 nm) and violet-blue (405 nm) photobiomodulation potentiality on human osteoblasts and mesenchymal stromal cells: A morphological and molecular in vitro study. Int J Mol Sci. 2018;19(7):1–23. PubmedPMID: 29970828.
- Peng F, Wu H, Zheng Y, Xu X, Yu J. The effect of noncoherent red light irradiation on proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells. Lasers Med Sci. 2012;27(3):645–53. Pubmed PMID: 22016038.
- Paschalidou M, Athanasiadou E, Arapostathis K, Kotsanos N, Koidis PT, Bakopoulou A, et al. Biological effects of low-level laser irradiation (LLLI) on stem cells from human exfoliated deciduous teeth (SHED). Clin Oral Investig. 2020;24(1):167–80. PubmedPMID: 31069538.
- Turrioni AP, Basso FG, Montoro LA, Almeida LFD, de Souza Costa CA, Hebling J. Transdentinal photobiostimulation of stem cells from human exfoliated primary teeth. Int Endod J. 2017;50(6):549–59.PubmedPMID: 27238557.
- Turrioni APS, Basso FG, Montoro LA, de Fátima D, de Souza Costa CA, Hebling J. Phototherapy up-regulates dentin matrix proteins expression and synthesis by stem cells from human-exfoliated deciduous teeth. J Dent. 2014;42(10):1292–9. Pubmed PMID: 25064041.
- Sivakumar TT, Muruppel AM, Joseph AP, Reshmi A, Ramachandran R, Nair PD, et al. Photobiomodulatory effect delivered by low-level laser on dental pulp stem cell differentiation for osteogenic lineage. Lasers Dent Sci. 2019;3(3):175–81.
- Hashmi JT, Huang YY, Sharma SK, Kurup DB, De Taboada L, Carroll JD, et al. Effect of pulsing in low-level light therapy. Lasers Surg Med. 2010;42(6):450–66. Pubmed PMID: 20662021.
- Kim HB, Baik KY, Choung PH, Chung JH. Pulse frequency dependency of photobiomodulation on the bioenergetic functions of human dental pulp stem cells. Sci Rep. 2017 Nov 21;7(1):15927. Pubmed PMID: 29162863.
- Tuby H, Maltz L, Oron U. Low-level laser irradiation (LLLI) promotes proliferation of mesenchymal and cardiac stem cells in culture. Lasers Surg Med Off J Am Soc Laser Med Surg. 2007;39(4):373–8. Pubmed PMID: 17457844.
- Pereira AN, Eduardo C de P, Matson E, Marques MM. Effect of low-power laser irradiation on cell growth and procollagen synthesis of cultured fibroblasts. Lasers Surg Med Off J Am Soc Laser Med Surg. 2002;31(4):263–7. Pubmed PMID: 12355572.
- . Oron U, Ilic S, De Taboada L, Streeter J. Ga-As (808 nm) laser irradiation enhances ATP production in human neuronal cells in culture. Photomed Laser Surg. 2007 Jun;25(3):180-2. Pubmed PMID: 17603858.
- Huertas RM, Luna-Bertos ED, Ramos-Torrecillas J, Leyva FM, Ruiz C, García-Martínez O. Effect and clinical implications of the low-energy diode laser on bone cell proliferation. Biol Res Nurs. 2014 Apr;16(2):191-6. Pubmed PMID: 23559459.
- Crisan L, Soritau O, Baciut M, Baciut G, Crisan BV. The influence of laser radiation on human osteoblasts cultured on nanostructured composite substrates. Clujul Med. 2015;88(2):224-32. Pubmed PMID: 26528075.
- Hakki SS, Bozkurt SB. Effects of different setting of diode laser on the mRNA expression of growth factors and type I collagen of human gingival fibroblasts. Lasers Med Sci. 2012;27(2):325–31. Pubmed PMID: 21246387.
- Ueda Y, Shimizu N. Effects of pulse frequency of low-level laser therapy (LLLT) on bone nodule formation in rat calvarial cells. J Clin Laser Med Surg. 2003;21(5):271–7. Pubmed PMID: 14651794.
- Keshri GK, Gupta A, Yadav A, Sharma SK, Singh SB. Photobiomodulation with Pulsed and Continuous Wave Near-Infrared Laser (810 nm, Al-Ga-As) Augments Dermal Wound Healing in Immunosuppressed Rats. PLoS One. 2016 Nov 18;11(11):e0166705. Pubmed PMID: 27861614.
- Ando T, Xuan W, Xu T, Dai T, Sharma SK, Kharkwal GB, et al. Comparison of therapeutic effects between pulsed and continuous wave 810-nm wavelength laser irradiation for traumatic brain injury in mice. PLoS One. 2011;6(10):e26212. Pubmed PMID: 22028832.
- Lapchak PA, Salgado KF, Chao CH, Zivin JA. Transcranial near-infrared light therapy improves motor function following embolic strokes in rabbits: an extended therapeutic window study using continuous and pulse frequency delivery modes. Neuroscience. 2007;148(4):907–14. PubmedPMID: 17693028.
- Joensen J, Øvsthus K, Reed RK, Hummelsund S, Iversen VV., Lopes- Martins RÁB, et al. Skin penetration time-profiles for continuous 810nm and superpulsed 904nm lasers in a rat model. Photomed Laser Surg. 2012;30(12):688–94. Pubmed PMID: 23025702.
- Bayat M, Azari A, Golmohammadi MG. Effects of 780-nm low-level laser therapy with a pulsed gallium aluminum arsenide laser on the healing of a surgically induced open skin wound of rat. Photomed Laser Surg. 2010;28(4):465–70. Pubmed PMID: 19795994.
- Karu TI. Cellular and molecular mechanisms of photobiomodulation (lowpower laser therapy). IEEE J Sel Top Quantum Electron. 2013;20(2):143–8.