Biocompatible Luminescent Nanosized Curcumin: Verified Parameters Affecting Stability and Bioavailability
Mostafa M. Mohamed1*, Hanaa S. Raslan1, Omneya R. Ramadan1, Salma T. Rafik2, Ashraf K. Awaad3,4, Marwa M. Essawy1,4
1 Oral Pathology Department, Faculty of Dentistry, Alexandria University, Alexandria, Egypt.
2 Clinical Pharmacology Department, Faculty of Medicine, Alexandria University, Alexandria, Egypt.
3 Biochemistry Department, Faculty of Science, Ain Shams University, Cairo, 21521, Egypt.
4 Center of Excellence for Research in Regenerative Medicine and Applications (CERRMA), Faculty of Medicine, Alexandria University, Alexandria, Egypt.
*Corresponding Author
MostafaM. Mohamed,
Faculty of Dentistry, Champollion Street, Azarita, Alexandria, Egypt.
Tel: +201224700660
E-mail: mostafa.mohamed.dent@alexu.edu.eg
Marwa M. Essawy,
Faculty of Dentistry, Champollion Street, Azarita, Alexandria, Egypt.
Tel: +201143847307
E-mial: marwa.morsy@alexu.edu.eg
Received: November 06, 2020; Accepted: December 05, 2020; Published: December 10, 2020
Citation:Mostafa M. Mohamed, Hanaa S. Raslan, Omneya R. Ramadan, Salma T. Rafik, Ashraf K. Awaad, Marwa M. Essawy. Biocompatible Luminescent Nanosized Curcumin:
Verified Parameters Affecting Stability and Bioavailability. Int J Dentistry Oral Sci. 2020;7(12):1204-1210. doi: dx.doi.org/10.19070/2377-8075-20000238
Copyright: MostafaM. Mohamed, Marwa M. Essawy©2020. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Purpose: Curcumin one of the herbal compounds that are used as an antitumor, antioxidant, and anti-inflammatory agent. However,
curcumin applications are limited due to its poor chemical stability and low water-solubility. On the contrary, the enhanced
solubility of nanocurcumin improves its bioavailability and cellular uptake. The present study aims to verify a simple, achievable,
and reproducible method for preparation of biocompatible nanocurcumin and to optimize the different parameters included in
this method.
Methods: In our study, we used solvent anti-solvent precipitation method to synthesis curcumin nanoparticles. For biocompatibility,
we screened different organic solvents to verify the best safe one with the highest solubility effect to curcumin. Furthermore,
we tested the effect of adding stabilizersto enhance the stability and yielding capacity of the nanocurcumin. Thereafter, the
stabilized nanosample was tested for water-solubility and luminescence.
Results: Acetone-dissolved nanocurcumin gave clear amber yellow nanosuspension without any precipitate and with an acceptable
concentration of nanosuspension. With limiting the stirring rate and time to the minimum, the nanosample was monodispersed.
Furthermore, stabilized nanocurcumin revealed the highest solubility and luminescence.
Conclusion: The prompt fluorescent property of the soluble nanosized curcumin would qualify this herbal nano-candidate to be
utilized as a theranostic agent.
2.Introduction
3.Background
4.Objective and Methodology
5.Analysis
6.Conclusion
7.Refereces
Keywords
Nanocurcumin; Solvent Anti-Solvent; Water-Solubility; Luminescence; Theranostic.
Introduction
Nanotechnology has entered the field of dentistry, resulting in a
magnitude evolution in dental materials and innovations in oral
health-related diagnostic and therapeutic methods. Thematerials
at a nanometric scale (10-9 cm) has unique physical, optical, and
electrical properties differ from their bulk form [1]. Inorganic nanoparticles
have been frequently investigated in the diagnosis and
treatment of oral cancer [2-4]. However, due to the reported toxicity
of metallic nanoparticles, biocompatible natural polymeric
nanoparticles have gained attention.Moreover, there is an increasing
demand for a safe nano-candidate that could be used dually as
theranostic agent [5].
Curcumin -a member of the ginger family- is a natural polyphenol
widely used asan herbal supplement. Biomedically, curcumin is
widely used as an anti-inflammatory and antimicrobial agent due
to its potent antioxidative activity. Moreover, curcumin exhibits
a unique anticancer activity through the induction of apoptosis,
inhibition of proliferation, and prevention of invasion, without delirious effect on the adjacent healthy cells [6-8].
Our team is interested in using herbal medicine, including curcumin,
in combating oral squamous cell carcinoma in vivo. However,
curcumin has shown limited bioavailability, clinical efficacy,
and low cellular uptake [9]. The poor water-solubility of the curcuminas
well as low chemical stability, consider as major obstacles
hindering its biological use. The curcumin molecule, due to its hydrophobicity,
tends to bind to the phospholipid of the cell membrane
by hydrogen bond, resulting in low availability of curcumin
inside the cytoplasm [10, 11]. Despite curcumin is soluble in different
organic solvents, the reported toxicity of these solvents
limits their biological uses.Therefore, curcumin water-solubility is
still the target for biocompatibility and safety purposes. One of
the most promising solutions for curcumin hydrophobicity is synthesizing
curcumin at nano-size [12]. Moreover, the water-soluble
nano-sized curcumin exhibits characteristicluminescent optical
properties that can be safely utilized in diagnostic nanomedicine
[13].
The solvent anti-solvent precipitation method is one of thepromising
bottom-up approaches for nanocurcumin preparation.
Curcumin nanoparticles precipitate as a result of the difference
of saturation caused by mixing the solution and the anti-solvent.
The way for producing nanoparticles by solvent anti-solvent precipitation
is to create conditions that enhance very rapid particle
formation without or little particle growth. The main advantages
of this approach are the simplicity, cost-effectiveness, and theeasiness
to scale-up the nanoparticles ideally [14, 15]. However, the
solvent anti-solvent precipitation method has many variables that
constrain its reproducibility and affect the sample stability. For
example, there are different organic solvents, where curcumin
shows different solubility rates. Furthermore, there are reservations
on some organic solvents due to their toxicity and environmental
aspects concerning their recycling and separation from the
anti-solvent [15].
Our study, therefore, aims to optimize a simple, achievable, and
reproducible method for the preparation of curcumin nanoparticles.
We screened different organic solvents and stabilizers to
reach the most potent biocompatible one, with superior yielding
capacity and stability. We concluded that the smallest polyvinyl
pyrrolidone coated nanocurcumin revealed uppermost stability,
bioavailability, and auto-fluorescence.
Curcumin powder was purchased from Alpha Chemika (Mumbai,
India). The coating agents; polyvinylpyrrolidone (PVP; Mw
40,000) and polyethylene glycol (PEG; Mw 6000), as well ascell
staining chemicals; Hoechst (H6024-10ML) and fluorescein isothiocyanate
(FITC) were obtained from Sigma-Aldrich (St. Louis,
MO, USA). Dimethyl sulfoxide (DMSO) was obtained from Fisher
Scientific (Loughborough, UK). Acetone and all other reagents
were of analytical grade and used as received. The used deionized
water (DIH2O) used to was ultra-purified from Millipore Milli-Q
system (resistivity ~80 MΩ cm).
We prepared the curcumin nanoparticles by the solvent anti-solvent
precipitation technique toinvestigate the effect of different
parameters on size, dispersity, yielding capacity, and stability of
the synthesized curcumin nanoparticles.
First, we screened the solubility rate of curcumin in different
organic solvents by dissolving 10mg of curcumin powder in 1
ml of dichloromethane, ethanol, DMSO, and acetone. Then, the
curcumin solutions were added slowly dropwise to 15ml DIH2O
under stirring at room temperature (25°C), using different stirring
rates (500, 800, and 1000rpm) and times (1 and 5 min) [16].
After that, we assessed the effect of different stabilizers with different
ratios by adding 0.5% and 1%w/v of PVP and PEG to DIH2O
before adding the curcumin solution.Finally, the nanosuspensions
werefreeze-dried by (BachiLyovapor L-200,Switzerland)
[17].
The preliminary detection of synthesized curcumin nanoparticles
was carried out by UV–visible spectrophotometer (Nanodrop,
DeNovix, DS-11 FX+, US)with scanning theabsorbance spectra
in the range of 200–800nm [18].
The average particle size, polydispersity index (PDI), and particle
charge of curcumin nanoparticles were performed by the dynamic
light scattering technique using Zeta-seizer (Nano ZS, Malvern
Instruments, Worcestershire, UK), with adilution ratio of 1:6.
All measurements were carried out in triplicate and performed at
room temperature [16].
The morphology and size of the synthesized curcumin nanoparticles
were characterized by transmission electron microscope(TEM;
JOEL, JSM-6360LA, JAPAN) [19].
Fourier transforms infrared spectroscopy (FTIR)analysis of curcumin,
stabilizer, and nanocurcumin was performed using an
FTIR spectrometer (PerkinElmer Inc, Shelton, CT, USA).A spectrum
for each sample was recorded within therange of 4000-500
cm-1 [17].
For assessing water-solubility of the nanocurcumin versus curcumin
powder, we dissolved 10 mg oflyophilized nanocurcumin
powder in 1 ml DIH2O, with comparing the solubility of an
equivalent dose of curcumin powder. Furthermore, we assessed
the nanocurcumin solubility in Dulbecco's Modified Eagle's Medium
(DMEM) [20].
To reach the extinction coefficient (ε) of the stabilized nanocurcumin,
we performed the standard curve, by preparing serial
concentrations of (5, 4, 3, 2, and 1 mg/ml) DIH2O-soluble nanocurcumin.
For plotting the standard curve of the curcumin nanoparticles,
UV–visible spectrophotometer was done to obtain the
absorption of each dilution. Then the absorbance (λ) was plotted
on the y-axis and concentration (c) on the x-axis to gain the equation
of Beer-Lambert law (c = λ/ε) [21].
The Alexandria University Ethics Committee approved the study
and the procedures followed are under the institutional guidelines
(IRBNO:00010556-IORG0008839). The photoluminescence of
water-soluble nanocurcumin was recordedby spectrofluorometer
(Nanodrop, DeNovix, DS-11 FX+, US) at specified excitation
wavelengths;470, 525, and 635nm to determine the absorption
at each wavelength. To confirm the emission wavelength of the
nanocurcumin, flow cytometric analysis was made at 488nm blue
laser by flow cytometry (BD Biosciences, Germany). We prepared
1ml of DIH2O-soluble nanocurcumin of 3 mM [13].
For in vitro visualization of the curcumin nanoparticles, gingival
fibroblast cells were seeded on cover glasses in a 6-well plate at
the density of 5x104 cells per well. Cells were treated with 50 μM
nanocurcumin dissolved in DMEM for 4 hours at 37°C in a 5%
CO2 incubator. Cells treatment with an equivalent concentration
of FITCwas used as positive control. The cells were fixed with
4% paraformaldehyde, after which they were permeabilized by
Triton X (0.2%). Finally, the cells were stained by Hoechst and
mounted on glass slides. We examined the cells under a confocal
laser scanning microscope (Leica TCS SPE, Germany) equipped
with imaging software (Leica LASX, Germany). Then, we analyzed
the intensity of fluorescence morphometrically using an
image analysis software (Image J; 1.52p software 32, NIH, USA)
[22].
We used IBMSPSSsoftwarepackage version 19.0 (IBM Inc., Chicago
IL, USA) to analyze the data. Considering normally distributed
variables, one-way ANOVA and Tukey multiple range tests
were conducted.Data are described as mean ± standard deviation
(SD) and the significant difference is determined at P-values <
0.05.
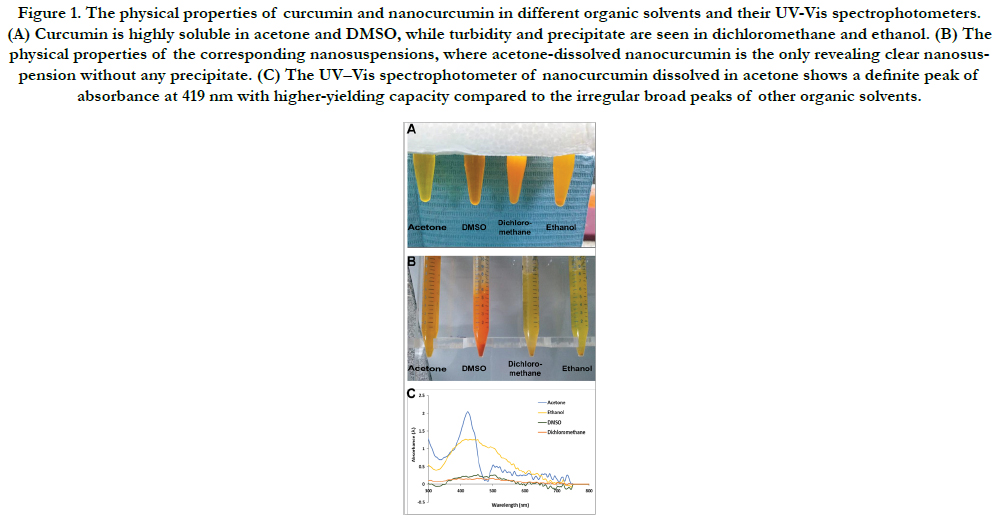
In our screening of the solubility rate of curcumin in different
organic solvents, curcumin powder was highly soluble in acetone
and DMSO, giving clear amber yellow and deep-orange solutions,
respectively. On the other hand, curcumin was less soluble in
dichloromethane and ethanol. Bothsolvents gave turbid yelloworange
solutions with precipitate (Fig. 1A).
Upon addition of the curcuminsolutions dropwise to DIH2O
under stirring, the resulted nanosuspensions were variable. The
DMSO revealed an immediate deep-orange precipitate, while dichloromethane
and ethanol gave yellow precipitate. On the other
hand, acetone gave clear amber yellow nanosuspension, without
any precipitate, (Fig. 1B).
Figure 1. The physical properties of curcumin and nanocurcumin in different organic solvents and their UV-Vis spectrophotometers. (A) Curcumin is highly soluble in acetone and DMSO, while turbidity and precipitate are seen in dichloromethane and ethanol. (B) The physical properties of the corresponding nanosuspensions, where acetone-dissolved nanocurcumin is the only revealing clear nanosuspension without any precipitate. (C) The UV–Vis spectrophotometer of nanocurcumin dissolved in acetone shows a definite peak of absorbance at 419 nm with higher-yielding capacity compared to the irregular broad peaks of other organic solvents.
Fig. 1C shows the UV–visible spectrophotometer of acetonedissolved nanocurcumin with a narrow smooth regular peak and specific absorbance at 419nm, which is the characteristic peak of nanocurcumin. Moreover, curcumin-acetone yielding capacity was higher than other different organic solvents. Meanwhile, nanocurcumin dissolved in different organic solvents (DMSO, dichloromethane, and ethanol) reveal irregular broad peaks without specific absorbance, reflecting non homogeneous nano-populations.
Consequently, in our next trial to optimize the time and rate of stirring, we focused on the acetone-suspended nanocurcumin.
In our trial optimizing time and rate of stirring, the synthesized
nanocurcumin particles were mono-modal dispersed, with an average size of 160.3 ± 4.4 nm and a low PDI of 0.3 ± 0.02, on
stirring power 500 rpm for 1 min. With increasing time of stirring
to 5 min and rate to 800 and 1000 rpm, the average size increased
significantly to 230.1 ± 11.5 nm and 242.5 ± 14.1 nm, respectively
(P<0.001). Furthermore, the nanoparticles clumped and became
multimodal dispersed, with significant increases of PDI to 0.5 ±
0.04 at 800 rpm and 0.4 ± 0.1 at 1000 rpm (P<0.05). Therefore,
in our attempts to increase the stability of nanosuspension, we
limited the stirring rate to 500 rpm and stirring time for 1 min.
As a result of the nonhomogeneous distributedcurcumin nanoparticles,
we used coating agents to achieve more stability and superior
yielding capacity. We compared two of the most intensely
used pharmaceutically polymers; PVP and PEG, at different ratios.
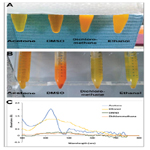
Stabilization with 0.5% PVP on stirring (1 min and 500 rpm)
gave a clear yellow solution without any precipitate. While PEG
with an equivalent ratio gave a turbid yellow suspension with precipitate,
(Fig. 2A).
Figure 2B shows the superior UV–visible spectrophotometer
results of 0.5% PVP stabilized nanocurcumin over PEG stabilized
nanoparticles at the equivalent ratio. The former revealed
a narrow, smooth regular peak with high yielding capacity at the
characteristic absorbance peak, of nanocurcumin at 419 nm. The
coating of curcumin nanoparticles with 0.5% PVP decreased the
average size significantly to 121 ± 0.03 nm (P<0.001). Furthermore,
it resulted in a homogenous monodispersed nanoparticles
population, with low PDI of 0.2 ± 0.02 and surface charge of
-12.9 mV, indicating the stabilization of nanosample, (Fig.2C-D).
Meanwhile, the PEG-coated nanocurcumin was nonhomogenous
with significant increases of the size to 343.9 ± 16.1 nm and PDI
to 0.4 ± 0.1, with a low surface charge to -4.26 mV (P<0.001, Fig.
2E-F).
Figure 2. The physical properties, UV-Vis spectrophotometer, and dynamic light scattering results of stabilized nanocurcumin with different concentrations of PVP and PEG. (A-D) Physically, 0.5% PVP gives well-stabilized nanosuspension, in contrast to the precipitate retrieved from PEG coating. Moreover, the PVP-stabilized nanocurcumin shows regular specific absorbance peak with higher-yielding capacity compared to the broad peak of PEG. Furthermore, the nano-sizer and potential results ensures the monomodal uniformly distributed PVP-stabilized nanosuspension, with the smallest particle size, compared to the multimodal dispersion with increased particle size retrieved from 0.5% PEG in (E-F). Increasing the concentration of PVP to 1% does not show improvements either in decreasing or homogenizing the resulted nanoparticles in (G-H).
With increasing the ratio of PVP to 1%, the retrieved nanopopulation was a nonhomogenous multimodal dispersed, with significant increases of the size to 559.5 ± 49.9 nm and PDI to 0.6 ± 0.08 (P<0.001, Fig.2 G-H). Furthermore, the surface charge decreased to -0.607 mV, indicating low stability of the nanopopulation.
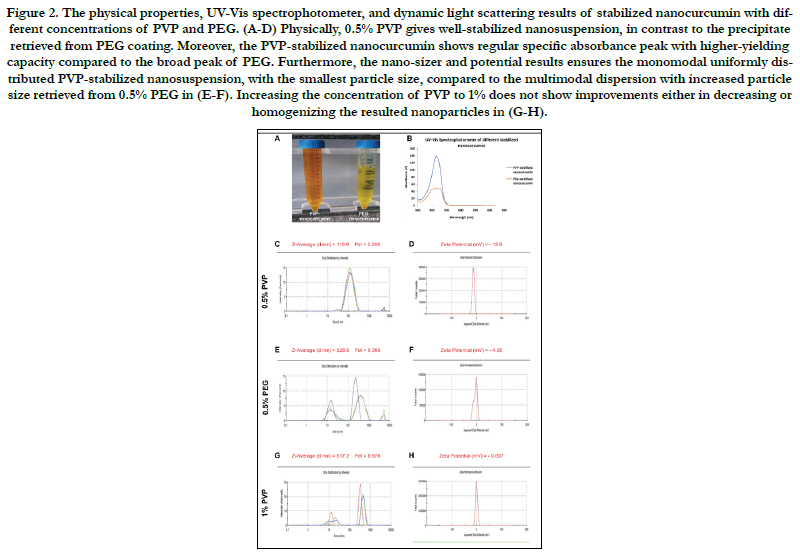
The TEM analysis of PVP-stabilized nanoparticles, showed round monodispersed particles with a mean size of 33.33 ± 10.1nm, (Fig. 3A). Furthermore, the FTIR results confirmed the success of synthesizing biocompatible PVP-coated nanocurcumin. Initially, comparing the simple shift in the absorbance spectrum between curcumin powder and nanoparticles in Fig. 3B indicates minor structural changes that occur during the solvent anti-solvent technique. Moreover, the absence of any peaks for acetone confirms the biocompatibility and the total purification of the synthesized dry nanocurcumin. On the other hand, the strong peaks in the ranges of 1651,1422, and 1290 cm-1 appeared in the nanocurcumin, compared to the corresponding ones in pure PVP, indicates the success in stabilizing the nanocurcumin.
In testing the solubility of the synthesized nanocurcumin, the PVP-stabilized nanoparticles were highly soluble in water and DMEM without any precipitate. In contrast, the solubility test proved the poor solubility of curcumin powder in water as well as in DMEM, (Fig. 3C).
Figure 3. The TEM, FTIR, solubility test, and the analytical curve of 0.5% PVP-stabilized nanocurcumin particles. (A) The TEM reveals spherical monodispersed particles. (B) Comparing between PVP-stabilized nanocurcumin and pure PVP, the FTIR results confirm the success of the coating process. (C) The solubility test reveals the high solubility of nanocurcumin versus curcumin in DIH2O and DMEM, which results in a highly concentrated nanosuspension of ~36.7 mM calculated from the analytical curve in (D).
According to the analytical curve in Fig. 4D, the PVP-stabilized nanosuspension revealed the superior yielding capacity of ~36.7 mM, compared to the initial 2.7 mM of curcumin powder. Consequently, the synthesis of curcumin at nano-size would help increasing its bioavailability and cellular uptake with improving its efficiency.br/>
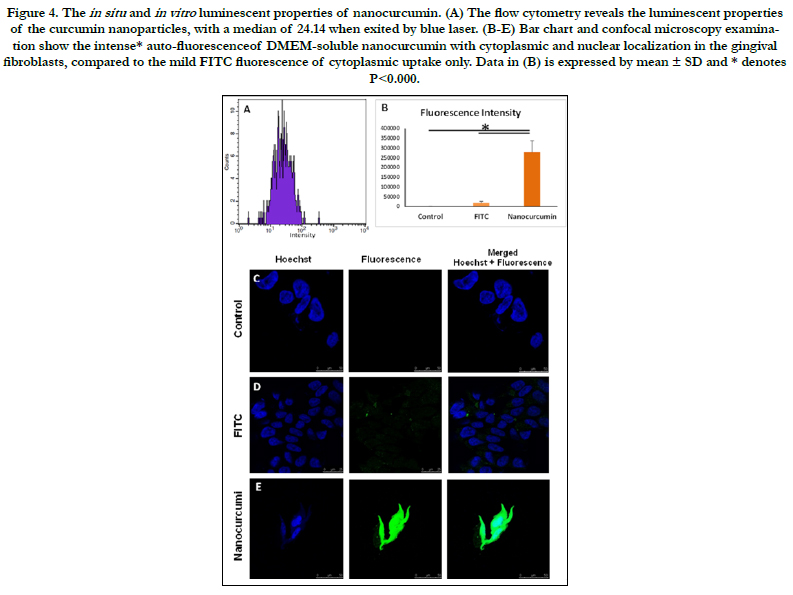
By the spectrofluorometer, the water-soluble nanocurcumin revealed
thehigh intensity of 165.351 RFU (relative fluorescence
unit) at the blue excitation. After determination of the excitationemission
spectra of curcumin nanoparticles, the flow cytometry
results confirmed the auto-fluorescence of a median 24.14 at the
similar excitation spectrum.
Interestingly, both nanocurcumin and FITC revealed their luminescence
at the blue excitation, upon confocal examination.
However, DMEM-soluble nanocurcumin showedintense fluorescenceof
a 1553.7-fold than the FITC (P<0.000). Moreover, the
luminescent nanoparticles showed nuclear and cytoplasmic localization,
where FITC localized in the cytoplasm of the gingival
fibroblast (Fig. 4).
Figure 4. The in situ and in vitro luminescent properties of nanocurcumin. (A) The flow cytometry reveals the luminescent properties of the curcumin nanoparticles, with a median of 24.14 when exited by blue laser. (B-E) Bar chart and confocal microscopy examination show the intense* auto-fluorescenceof DMEM-soluble nanocurcumin with cytoplasmic and nuclear localization in the gingival fibroblasts, compared to the mild FITC fluorescence of cytoplasmic uptake only. Data in (B) is expressed by mean ± SD and * denotes P<0.000.
Discussion
Curcumin, as a natural compound widely used in herbal medicine,
has a decreased biomedical efficiency due to its poor water-solubility.
Nanotechnology has been used to overcome the problems
of decreased stability and bioavailability associated with poorly
soluble drugs. Wesettled on the solvent anti-solvent precipitation
method, which is suitable for the synthesis of highly soluble
nanocurcumin. However, this approach has many variables that
directly influence the particle size, stability, and dispersity of the
resulted nanopopulation.
From our screening of curcumin solubility in various organic solvents,
acetone was verified as the best solvent. Biologically, acetone
is safe and can be easily removed from the final formulation
by evaporation due to its low boiling point 56°C [16]. Physically,
curcumin is highly soluble in acetone, giving a clear yellow solution
without any precipitate. Our UV–visible spectrophotometer
results confirmed the synthesis of curcumin nanoparticles from
acetone-dissolved curcumin solution with an absorbance peak of
419nm, following the results of Alam et al., [23] and Ghosh et
al., [24].
Stirring rate and time are pivotal factors affecting sample stability,
dispersity, and size. In the present study, the stirring rate at 500
rpm for 1 min was significantly better than the other increased
stirring rates(800 and 1000 rpm) for 5 min. In our verification,
slow stirring rate was gentle enough to complete the mixing of
curcumin-acetone solution with the anti-solvent. Therefore, the
particle growth retained apart from each other at a smaller size,
resulting in a monodispersed nanopopulation. Meanwhile, the
aggressive mixing with an increased duration resulted in clump
and collisions of the curcumin nanoparticles, promoting their agglomeration
and growth with wide particle size distribution [16].
Theuse of stabilizers gained our attention to obtain homogeneously
distributed nanopopulation of small size. We compared two
of the well-known coating agents; PVP and PEG. They provide
corona surrounding the nanoparticles preventing their precipitation
[25]. However, it was still critical to verify the appropriate
amount of stabilizer needed to achieve the optimized nanoparticles’
surface coverage ratio.
Upon stabilizing the nanocurcumin with 0.5% PVP, the size decreased
significantly, and the nanoparticles became homogeneously
distributed. The PVP migrates immediately to the hydrophobic
surface of the newly formed curcumin nanoparticles and
prevent their growth, resulting in small particle size. On the contrary,
coating the nanocurcumin with 0.5% PEG did not reduce
the size as PVP at an equivalent ratio. Moreover, it resulted in
a poly-dispersed nanopopulation, with broad size distribution.
These might be due to the difference in the molecular weight of
the used stabilizers, where PEG was 6000, while PVP was 40,000.
Increasing the molecular weight of the stabilizer would increase
its flexibility and elasticity, which in turn improves the ability of
the stabilizer to surround and make a full inclusion of the newly
synthesized curcumin nanoparticles inside it [26]. On the other
hand, if the polymer length is too short, the nanoparticles would not be covered with the polymer layer completely, leading to their
agglomeration. Furthermore, the high hydrophobicity of the
PEG prevents the adsorption on the nanocurcumin particle surface
for steric stabilization [27, 28].
The 0.5% of PVP was sufficient to cover the whole nanoparticle
surface and to provide enough steric repulsion between them.
Upon increasing PVP concentration to 1%, the mean particle size
increased significantly. Similarly, several studies have reported the
increase of nanoparticle size, with increasing the amount of the
stabilizer [29-31]. The high concentration of the stabilizer would
form a thick polymer layer on the particles, contacting them mutually
and exacerbating the agglomeration of nanoparticles [32].
In another study, Yadav and his colleagues [16] have used gelatin
as a stabilizer in the synthesis of nanocurcumin. However, they
concluded that gelatin was effective in arresting the growth of nanocurcumin
butdid not prevent their aggregation. Gelatin created
a high negative surface charge (-30 mV) around the newly synthesized
curcumin nanoparticles, which prevents size growth but
allows the aggregation of particles. The PVP in the current study,
in contrast, formed an acceptable negative surface charge (-12.9
mV) around curcumin nanoparticles, preventing their growth as
well as their aggregation.
The proven water-solubility of synthesized PVP-stabilized nanocurcumin
would enhance its bioavailability and cellular uptake,
with improving its clinical efficacy. Moreover, the intense autofluorescence
of the synthesized PVP-stabilized nanocurcumin
would help the in-situ tracking of the curcumin nanoconstruct at
the cellular level. Mogharbel et al., [13] have utilized the fluorescence
properties of curcumin-loaded nanoparticles for tracking
cellular therapy in regenerative nanomedicine.Depending on this
optical property of the curcumin nanoparticles the herbal nanomedicine
will have an added value in the diagnosis besides medication
[33, 34].
From our results, we concluded that nanosized curcumin precipitated
by stirring acetone-dissolved curcumin and DIH2O at aslow
rate and short time was uniformly dispersed population of high
yielding capacity. Furthermore, asmall amount of high molecular
weight stabilizer reveals the high stability. Moreover, luminescent
property of water/DMEM-soluble nanocurcumin would qualify
this herbal nano-candidate to serve as a double theranostic agent,
maximizing benefits of one-step therapies.
Acknowledgement and Declarations
We express our deep gratitude to the teams of the Medical Nanotechnology
Lab and Tissue Culture Lab in the Center of Excellence
for Research in Regenerative Medicine and Applications
(CERRMA; STDF-Funded), Faculty of Medicine, Alexandria
University for providing the technical help during the conduction
of the study.
References
- Sweeney AE. Nanomedicine concepts in the general medical curriculum: initiating a discussion. Int J Nanomedicine. 2015 Dec 7;10:7319-31.Pubmed PMID: 26677322.
- Essawy MM, El-Sheikh SM, Raslan HS, Ramadan HS, Kang B, Talaat IM, et al. Function of gold nanoparticles in oral cancer beyond drug delivery: Implications in cell apoptosis. Oral Dis. 2020 Jul 13.Pubmed PMID: 32657515.
- Afifi MM, Austin LA, Mackey MA, El-Sayed MA. XAV939: from a small inhibitor to a potent drug bioconjugate when delivered by gold nanoparticles. Bioconjug Chem. 2014 Feb 19;25(2):207-215.Pubmed PMID: 24409808.
- Afifi MM, El Sheikh SM, Abdelsalam MM, Ramadan H, Omar TA, El Tantawi M, et al. Therapeutic efficacy of plasmonic photothermal nanoparticles in hamster buccal pouch carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013 Jun;115(6):743-51.Pubmed PMID: 23454046.
- Sukhanova A, Bozrova S, Sokolov P, Berestovoy M, Karaulov A, Nabiev I. Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res. Lett. 2018 Dec 1;13(1):44.
- Dai C, Ciccotosto GD, Cappai R, Tang S, Li D, Xie S, et al. Curcumin Attenuates Colistin-Induced Neurotoxicity in N2a Cells via Anti-inflammatory Activity, Suppression of Oxidative Stress, and Apoptosis. Mol Neurobiol. 2018 Jan;55(1):421-434.Pubmed PMID: 27957686.
- Kunnumakkara AB, Bordoloi D, Padmavathi G, Monisha J, Roy NK, Prasad S, et al. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharmacol. 2017 Jun;174(11):1325-1348.Pubmed PMID: 27638428.
- Liu F, Gao S, Yang Y, Zhao X, Fan Y, Ma W, et al. Curcumin induced autophagy anticancer effects on human lung adenocarcinoma cell line A549. Oncol Lett. 2017 Sep;14(3):2775-2782.Pubmed PMID: 28928819.
- Saleh MM, Darwish ZE, El Nouaem MI, Mourad GM, Ramadan OR. CHEMOPREVENTIVE EFFECT OF GREEN TEA AND CURCUMIN IN INDUCED ORAL SQUAMOUS CELL CARCINOMA: AN EXPERIMENTAL STUDY. Alex Dent J. 2020 Apr 15.
- Nagahama K, Utsumi T, Kumano T, Maekawa S, Oyama N, Kawakami J. Discovery of a new function of curcumin which enhances its anticancer therapeutic potency. Sci Rep. 2016 Aug 1;6:1-14.Pubmed PMID: 27476814.
- Tsukamoto M, Kuroda K, Ramamoorthy A, Yasuhara K. Modulation of raft domains in a lipid bilayer by boundary-active curcumin. Chem Commun (Camb). 2014 Apr 4;50(26):3427-30.Pubmed PMID: 24396862.
- Tønnesen HH, Karlsen J. Studies on curcumin and curcuminoids. Z Lebensm Unters Forsch. 1985 May 1;180(5):402-4.
- Mazzarino L, Lemos-Senna E, Borsali R, Soto PA, Setton-Avruj P, Abdelwahid E, et al. Fluorescence properties of curcumin-loaded nanoparticles for cell tracking. Int J Nanomedicine. 2018 Sep 28;13:5823-5836.Pubmed PMID: 30319253.
- Matteucci ME, Hotze MA, Johnston KP, Williams RO 3rd. Drug nanoparticles by antisolvent precipitation: mixing energy versus surfactant stabilization. Langmuir. 2006 Oct 10;22(21):8951-9.Pubmed PMID: 17014140.
- Viçosa A, Letourneau JJ, Espitalier F, Re MI. An innovative antisolvent precipitation process as a promising technique to prepare ultrafine rifampicin particles. J. Cryst. Growth. 2012 Mar 1;342(1):80-7.
- Yadav D, Kumar N. Nanonization of curcumin by antisolvent precipitation: process development, characterization, freeze drying and stability performance. Int J Pharm. 2014 Dec 30;477(1-2):564-77.Pubmed PMID: 25445971.
- Homayouni A, Sohrabi M, Amini M, Varshosaz J, Nokhodchi A. Effect of high pressure homogenization on physicochemical properties of curcumin nanoparticles prepared by antisolvent crystallization using HPMC or PVP. Mater. Sci. Eng: C. 2019 May 1;98:185-96.
- Pandit RS, Gaikwad SC, Agarkar GA, Gade AK, Rai M. Curcumin nanoparticles: physico-chemical fabrication and its in vitro efficacy against human pathogens. 3 Biotech. 2015 Dec;5(6):991-997. Pubmed PMID: 28324406; PMCID: PMC4624150.
- Dende C, Meena J, Nagarajan P, Nagaraj VA, Panda AK, Padmanaban G. Nanocurcumin is superior to native curcumin in preventing degenerative changes in Experimental Cerebral Malaria. Sci Rep. 2017 Aug 30;7(1):1-12. Pubmed PMID: 28855623.
- Abirami M, Raja J, Mekala P, Visha P. Preparation and characterisation of nanocurcumin suspension. Int J Environ Sci Technol. 2018;7(1):100-3.
- Kadam PV, Yadav KN, Bhingare CL, Patil MJ. Standardization and quantification of curcumin from Curcuma longa extract using UV visible spectroscopy and HPLC. Int. J. Pharmacogn. Phytochem. 2018;7(5):1913-8.
- Awaad AK, Kamel MA, Mohamed MM, Helmy MH, Youssef MI, Zaki EI, et al. The role of hepatic transcription factor cAMP response element-binding protein (CREB) during the development of experimental nonalcoholic fatty liver: a biochemical and histomorphometric study. Egypt. Liver J. 2020 Dec;10(1):1-13.
- Alam S, Panda JJ, Chauhan VS. Novel dipeptide nanoparticles for effective curcumin delivery. Int J Nanomedicine. 2012;7:4207-22.Pubmed PMID: 22915849.
- Ghosh M, Singh AT, Xu W, Sulchek T, Gordon LI, Ryan RO. Curcumin nanodisks: formulation and characterization. Nanomedicine. 2011 Apr;7(2):162-7.Pubmed PMID: 20817125.
- Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanoparticle PEGylation for imaging and therapy. Nanomedicine. 2011 Jun;6(4):715-28.
- Obaidat R, Al-taani B, Al-quraan HA. Effect of selected polymer on dissolution and stabilization of amorphous form of meloxicam. Int J Pharm Pharm Sci. 2017;9(9):33-42.
- Hecold M, Buczkowska R, Mucha A, Grzesiak J, Rac-Rumijowska O, Teterycz H. The effect of PEI and PVP-stabilized gold nanoparticles on equine platelets activation: potential application in equine regenerative medicine. J. Nanomater. 2017 Jan 1;2017.
- Alshora DH, Ibrahim MA, Elzayat E, Almeanazel OT, Alanazi F. Rosuvastatin calcium nanoparticles: Improving bioavailability by formulation and stabilization codesign. PLoS One. 2018 Jul 9;13(7):e0200218.Pubmed PMID: 29985967.
- Blagden N, de Matas M, Gavan PT, York P. Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Adv Drug Deliv Rev. 2007 Jul 30;59(7):617-30.Pubmed PMID: 17597252.
- Bozkir A, Saka OM. Formulation and investigation of 5-FU nanoparticles with factorial design-based studies. Farmaco. 2005 Oct;60(10):840-6.Pubmed PMID: 16087183.
- Branham ML, Moyo T, Govender T. Preparation and solid-state characterization of ball milled saquinavir mesylate for solubility enhancement. Eur J Pharm Biopharm. 2012 Jan;80(1):194-202.Pubmed PMID: 21906676.
- Xiliang Q, Yang C, Tiesong L, Peng H, Jun W, Ping L, et al. Large-scale synthesis of silver nanoparticles by aqueous reduction for low-temperature sintering bonding. J. Nanomater. 2014 Jan 1;2014.
- Basniwal RK, Buttar HS, Jain VK, Jain N. Curcumin nanoparticles: preparation, characterization, and antimicrobial study. J Agric Food Chem. 2011 Mar 9;59(5):2056-61.
- Kesisoglou F, Panmai S, Wu Y. Nanosizing--oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev. 2007 Jul 30;59(7):631-44.Pubmed PMID: 17601629.