Distribution of Fc Gamma Receptor IIa Genotypes among Malay Patients with Periodontitis
Ayros-Asmawati Mohd Ayub1, Haslina Taib2*, Azlina Ahmad3, Khairani Idah Mokhtar@Makhtar4, Siti LailatulAkmar Zainuddin2
1 Periodontist Specialist Clinic, Jalan Sultan Mahmud Dental Clinic, 20400 Kuala Terengganu, Terengganu, Malaysia.
2 Periodontics Unit, School of Dental Sciences, Hospital Universiti Sains Malaysia, Health Campus Universiti Sains Malaysia, Kubang Kerian, 16150
Kota Bharu, Kelantan, Malaysia.
3 Biochemistry Unit, School of Dental Sciences, Health Campus Universiti Sains Malaysia, Kubang Kerian, 16150 Kota Bharu, Kelantan, Malaysia.
4 Kuliyyah of Dentistry, International Islamic University, Kuantan Campus, Jalan Sultan Ahmad Shah, Bandar InderaMahkota, 25200 Kuantan, Pahang,
Malaysia.
*Corresponding Author
Dr. Haslina Taib,
Associate Professor, Periodontics Unit, School of Dental Sciences, UniversitiSains Malaysia, Health Campus, Kubang Kerian, 16150 Kota Bharu, Kelantan, Malaysia.
Tel: +6 09 7675812
Fax: +6 09 7675505
E-mail: haslinakk@usm.my
Received: October 07, 2020; Accepted: November 06, 2020; Published: November 12, 2020
Citation:Ayros-Asmawati Mohd Ayub, Haslina Taib, Azlina Ahmad, KhairaniIdahMokhtar@Makhtar, Siti Lailatul Akmar Zainuddin. Distribution of Fc Gamma Receptor IIa Genotypes among Malay Patients with Periodontitis. Int J Dentistry Oral Sci. 2020;7(11):1007-1011. doi: dx.doi.org/10.19070/2377-8075-20000200
Copyright: Haslina Taib©2020. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: To evaluate the distribution of Fc gamma receptor IIa (FcγRIIa) gene polymorphism and its possible association with
periodontitis in Malay patients.
Materials and Methods: Periodontal parameters were recorded in 53 periodontitis and 65 healthy (control) subjects. Samples for
genotype analysis were obtained from the buccal cheek mucosa. The FcγRIIa genotypes were determined via nested polymerase
chain reaction by using sequence specific primers.
Results: The FcγRIIa R/H131 genotype showed the highest prevalence (56.6% in periodontitis and 50.8% in control) by which
the H131 allele was slightly dominant in all subjects (51% in periodontitis and 52% in control). However, no significant association
was observed between the periodontal parameters and FcγRIIa genotypes (p>0.05).
Conclusion: Within the study limitations, FcγRIIa R/H131 genotype was mostly distributed in Malay subjects. There is no significant
association between FcγRIIa gene polymorphisms with periodontitis.
2.Introduction
3.Material and Methods
4.Results and Discussions
5.Conclusion
6.Acknowledgement and Declaration
7.Refereces
Keywords
Periodontitis; Fc Gamma Receptor IIa; Gene Polymorphism; Genotypes.
Introduction
Periodontitis is a chronic inflammatory disease affecting supporting
tissues of teeth such as the gingiva, periodontal ligament, connective
tissues, cementum and alveolar bone. Apart from bacterial
biofilm as the local etiological factor, impaired host immune response
towards bacterial infections also contribute to the disease
initiation and progression. Some studies have proposed the idea
of genetic risk factor and its subsequent relationship with periodontitis
[1-3]. Genetic risk factors have been underlined as the
causal chainsor expose the host to the causal chain to develop
periodontitis. In case of their absence, the possibility of developing
the disease may be reduced [4].
Fc is the constant part of antibodies, whereby its receptors provide
a crucial link between the cellular and humoral parts of the
immune system that confers the potent effector functions to the
antibody. In particular, immunoglobulin G (IgG) is the most
dominant antibody class in the plasma in which receptors for IgG
(FcγR) will trigger various effector functions such as phagocytosis,
antibody-dependent cellular cytotoxicity, antigen presentation,
cytokine release, degranulation, and regulation of antibody
synthesis. FcγR is expressed in a wide variety of cells, including
leukocytes from myeloid and lymphoid lineages, endothelial cells,
placental tissue, and the synovial and mesangial cells. It is also
found on a multitude of immune cells in the periodontal tissues
[5].
In leukocytes, FcγR belongs to the immunoglobulin superfamily
andmay be categorised in three main classes of FcγRI (CD64);
FcγRII (CD32); and FcγRIII (CD16). These classes are further
subdivided into subclasses: FcγRIa and Ib; FcγRIIa, IIb and IIc;
and FcγRIIIa and IIIb. When one or multiple FcγR-mediated
leukocyte functions are compromised or exaggerated due to genetic polymorphism in the FcγR genes, susceptibility to and/or
the severity of periodontitis are affected [4]. The structural and
functional differences in FcγRIIa, FcγRIIIa and IIIbas a result
of polymorphism in the genes have been described by many researchers
[4, 6-11].
Normally, the binding of FcγRIIa with IgG2 bound to antigens
results in phagocytosis, namely the killing of opsonised cellular
target via antibody-directed cell cytotoxicity and respiratory burst
[12]. FcγRIIa is the sole receptor on leukocytes that can interact
with IgG2 [13]. Meanwhile, IgG2 provides the humoral response
against polysaccharide antigens on the cell wall of Gram-negative
periodontal pathogens such as Aggregatibacter actinomycetemcomitans
[14]. In brief, efficient phagocytosis of pathogenic bacteria
by phagocytes via IgG2-FcγRIIa interactions is crucial for
the purpose of host defenses in periodontal infections.
The gene that codes for FcγRIIa has been known to have a single
nucleotide polymorphism (SNP), which is determined by the
presence of either histidine or arginine residue at the amino acid
position 131.Conversely, different genotypes will affect the binding
between the Fc part of the antibody IgG2 and opsonised
antigen to the FcγR. Periodontitis patients who are homozygous
for the H-allele (H/H 131 genotype) in FcγRIIa gene polymorphism
have been linked to more periodontal bone loss compared
to those carrying one or two R-alleles [9]. FcγRIIa genotypes have
been particularly and significantly associated with a severe case
ofchronic periodontitis, while the homozygosity for the H-allele
has resulted in a significant over-representation in periodontitis
smokers [10]. Furthermore, Kobayashi et al. have observed that
another genotype of FcγR, namely FcγRIIIa N-allele is over-represented
in subjects with severe periodontitis compared to those
with a moderate form of the disease [8]. Upon an assessment of
a group of Dutch subjects, FcγRIIIa N-allele (V158) has been
proposed as a risk factor for periodontitis, especially aggressive
periodontitis [9].
Different ethnic backgrounds carry different prevalence of FcγR
genotypes, which is a concept rooted based on previous studies
conducted [15]. This is evidenced by the higher carriage rate of
R-allele in FcγRIIa gene observed in Caucasians and African-
Americans [9, 10]. In contrast, such ratesare lower in Japanese
population [8]. Meanwhile, to date, no information onthe prevalence
of FcγRIIa genotypes in periodontitis patients in Malaysia.
Therefore, this study was conducted to determine the prevalence
of FcγRIIa genotypes and their association with periodontitis
among Malay subjects.
Materials and Methods
A cross-sectional study was conducted on 118 Malay patients
attending the Dental Clinic, Hospital UniversitiSains Malaysia
(USM), Kelantan, Malaysia. The criteria included were subjects
aged 30 to 65 years old with at least of six teeth are present not
including the wisdom teeth. The subjects were included if they
were diagnosed with generalised periodontitis and presented with
at least 30% (≥30%) of periodontal sites with probing pocket
depths (PPD) greater than 3mm, and evidences of alveolar crestal
bone loss in the orthopanthomogram radiograph (OPG) of 30%
or more sites.
The control group includes subjects who matched the age range
andhadno evidence of periodontal disease. Those with uncontrolled
medical problems, required prophylactic antibiotics prior
to any dental procedures, pregnancy, and received antibiotics
therapy within six months prior to the study were excluded. Subjects
were informed about the study and written informed consent
was obtained from each of them upon agreement of participation.
Clinical parameters, such as number of missing teeth,
plaque index (PI) [16], gingival index (GI) [17], probing pocket
depth (PPD), clinical attachment loss (CAL), and alveolar bone
loss (ABL) were subsequently recorded. The study protocol was
approved by the Human Research and Ethics Committee, USM
(USMKK/PPP/JEPeM [232.3.(04)].
DNA sample was collected from the buccal cheek mucosa by using
a sterile swab stick. The samples were stored in a -20°C freezer
until further use. Genomic DNA was isolated from the buccal
cells swab according to the manufacturer’s protocols with minor
modifications post-optimisation (GeneAllTM Blood SV, GeneAll
Biotechnology, Korea). The presence of DNA was ascertained
via gel electrophoresis (1%) and viewed under the image analyser
(Gel DocTM XR+ System, Bio Rad, USA).
Determination of FcγRIIa gene polymorphisms was undertaken
according to the Nested Sequence-Specific Primer-Polymerase
Chain Reaction (SSP-PCR) with minor modifications after optimization
[18]. The SSP-PCR method required the product obtained
from the first PCR amplification to be re-amplified using
a sequence specific primer. The primers for the first step of PCR
were designed using a primer pair previously published [19] as
follows:
i) sense primer P63 (5’-CAA GCC TCT GGT CAA GGT C) and,
ii) antisense P52 (5’-GAA GAG CTG CCC ATG CTG) primer
Meanwhile the second set of primers utilized, was designed by
Carlsson et al. [18] as follows:
i) sense primers of P5G (5’-GAA AAT CCC AGA AAT TTT
TCC G) or, P4A (5’-GAA AAT CCC AGA AAT TTT TCC A) –
the allele-specific bases are in bold and inserted mismatch bases
in the sequence are underlined.
ii) common antisense primer P13 (5’-CTA GCA GCT CAC CAC
TCC TC).
The first PCR required approximately 100ng of genomic DNA,
which was added to 25μL reaction mixes containing 50mM Tris (pH 8.4); 125mM KCl;
0.02mM MgCl2; 0.05mM of each dNTP;and 0.4μM each of
P63 (5’-CAA GCC TCT GGT CAA GGT C) and P52 (5’-GAA
GAG CTG CCC ATG CTG) primers. The mixes also contained
0.025μM each of the CRP primers (440bp) (upstream: CCA GCC
TCT CTC ATG CTT TTG GCC AGA CAG and downstream:
GGG TCG AGG ACA GTT CCG TGT AGA AGT GGA) as
the internal control and 0.025U of Taq DNA Polymerase (Invitrogen,
Brazil).
PCR conditions employed were set at 1 cycle at 95°C for 5 min,
55°C for 5 min, and 71°C for 5 min. This was followed by 35 cycles
of 95°C for 1 min, 60.4°C for 1 min, and 72°C for 2 min before ending with an extension step at 72°C for 10 min. The conditions
for the second PCR were as detailed: 95°C for 5 min and
followed by 30 cycles of 95°C for 15 s 58°C for 30 s, and 72°C
for 30 s, with an extension step at 72°C for 10 min. The PCR
products were analysed via electrophoresis on 2% agarose gel
stained with SYBR Green I Nucleic Acid Gel Stain. The FcγRIIa
gene was observed at 278-bp and distinguished as homozygous
or heterozygous accordingly by the presence or absence of the
band.Meanwhile, the internal control was assessed at 440-bp and
viewed under the UV imaging and documentation system (Gel
DocTM XR+ System, Bio Rad, USA).
Statistical analysis was performed using the Statistical Package for
the Social Sciences (SPSS) Version 20 for Windows. The descriptive
data was expressed by mean and standard deviation (SD) and,
frequency and percentage. The distribution of genotypes between
the two groups was further analysed by using the Chi-square test.
Furthermore, the association between the genotypes and periodontal
parameters (i.e. number of missing teeth, PI, GI PD, CAL,
and bone loss) in the periodontitis group was assessed using the
Kruskal-Wallis test as the data were not normally distributed. P
values <0.05 at 95% confidence interval (CI) were considered statistically
significant.
Results and Discussions
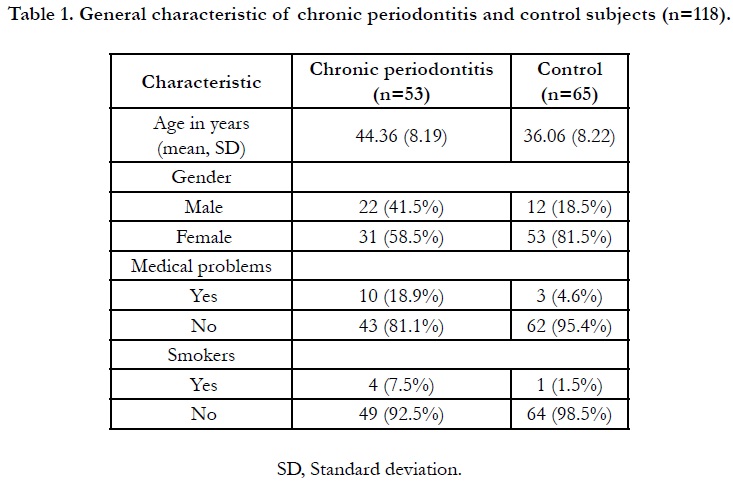
Overall, 118 subjects enrolled, 53 were periodontitis and 65 were
control subjects by which females being the majority (71.20%).
The mean age of periodontitis and control groups were 44.36
(SD8.19) and 36.06 (SD8.22) years oldrespectively. A portion
ofthe periodontitis subjects (19%) recorded to have medical problems,
such as hypertension, osteoarthritis and hypercholesterolemia,
whereas only hypertension was noted in the control group
(4.6%). However, the conditions were under-controlled. A small
number of the periodontitis subjects (7.5%) and control (1.5%)
were smokers. The general characteristics of the study subjects
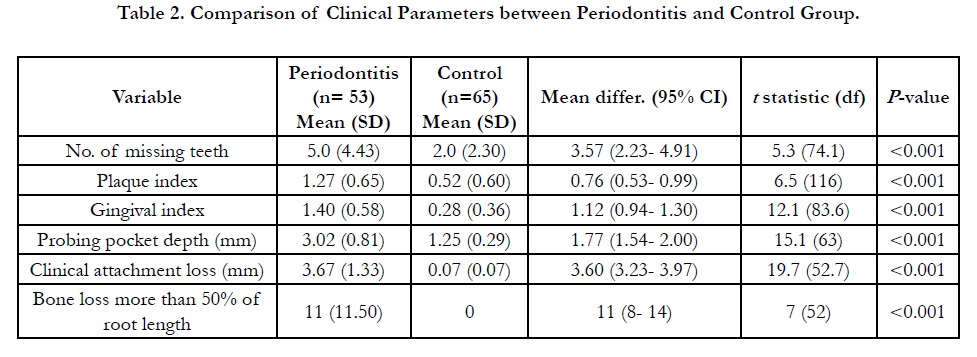
are shown in Table 1. All periodontal parameters were significantly
difference (p<0.05) between periodontitis and control subjects
(Table 2), thus justified the criteria for the control group.
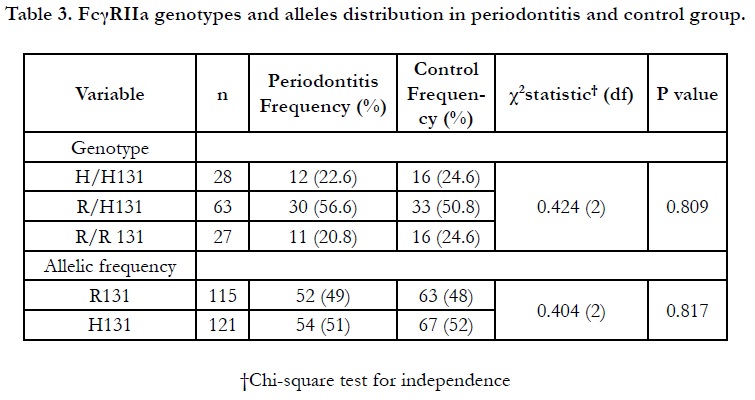
This present study observed that the distribution of FcγRIIa heterozygous R/H131 appeared as the most prevalent genotype in the total subjects (53.4% out of 118 subjects) as seen in Table 3. The allelic frequencies of R131 and H131 alleles were found to be almost equally distributed in periodontitis (51%) and control subjects (52%). Consistently, the result shows periodontitis and control subjects revealed a higher frequency of heterozygous R/H 131 carrier (56.6% and 50.8%, respectively) compared to other FcγRIIa genotypes. Such genotypic prevalence is in agreement with the local findings by Yap and co-workers among healthy and systemic lupus erythematous patients [11]. We also showed that there was no significant difference of genotype distribution between the periodontitis and control subjects (p=0.089).
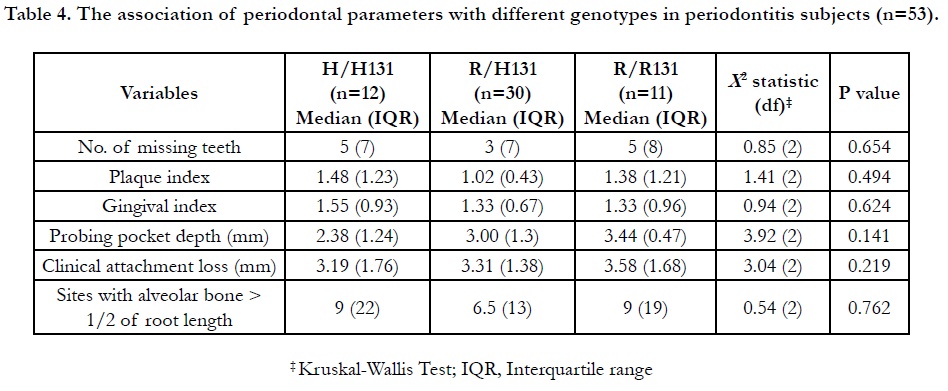
This current study shows that FcγRIIa gene polymorphisms were not found to be significantly associated with the periodontal parameters i.e. number of missing teeth, PI, GI, PPD, CAL, and ABL (Table 4). PPD and CAL were slightly higher in the R/R 131 genotypes, although not by a significant value. In terms of bone loss evaluation, the amount of sites having severe bone loss of more than half of root length was found to bealmost equally presented among the three genotypes. In contrast with previous study in Chinese Han population, the frequency of genotype R/ R131 was suggested as one of the contributors for the increased susceptibility of severe periodontitis as it was found to be significantly higher (P < 0.0125) compared to their healthy patients [20]. The prevalent of R/R genotype also have been demonstrated by the study on Iranian population with periodontitis and periimplantitis [21].
Table 4. The association of periodontal parameters with different genotypes in periodontitis subjects (n=53).
A study done among the Taiwanese population showed that the genotypes with at least one R-allele in the FcγRIIa gene polymorphism were over-presented in the generalised aggressive periodontitis subjects [22]. The study on South Indian population resulting in similar findings, with the R-allele being more prevalent in the population and was also significantly over-presented in aggressive periodontitis subjects [6]. A subsequent study on the same population but incorporating generalised periodontitis subjects had further found over-representation of R/R genotype [7]. Nevertheless, an insignificant difference in the distribution of genotypes in periodontitis and healthy control subjects was seen, which was consistent with the current study.
It was suggested that the measurement of in-vitro activity of FcγRIIa genotypes may not be related to the severity of the diseases as speculated, such as the hyper-responsive PMNs with FcγRIIa H/H131 genotype [12]. Both H131 and R131 alleles were almost equally present in periodontitis and controlsubjects, although H131 was slightly over-represented in this study. Furthermore, it was reported that the H131 allele was associated with poor receptor binding with IgG2 immune complex in-vitro [23]. Such feature could possibly explain the less severe pattern of periodontal destruction observed. However, another previous study assessing Caucasian smokers showed that the carriage rate of FcγRIIa H/H131 genotype was enriched and associated with more severe periodontal breakdown [10]. The work also concluded that the H/H 131 genotype and smoking had a synergistic effect. With respect to the population in this current study, a very small number of the periodontitis patients and controls were current smokers, thus rendering the association unjustifiable.
Genetic polymorphism studies aimed to evaluate the association with periodontal disease have been reported with having inconsistent findings. This is predictable due to the variations in ethnic groups, limited sample sizes, variable definitions used to define the disease, and covariates and risk factors that needed adjustments. Genotyping of FcγRIIa gene in periodontitis subjects have been conducted in some Asian countries. The distribution of FcγRIIa genotypes in a Malay population with periodontitis has never been reported despite existed in other populations. In Malaysia, the prevalence of these gene polymorphisms were demonstrated in a study of Malay patients in relation to systemic lupus erythematosus [11].
The results of the current study revealed a low carriage rate of the genotype expressing the hyper-responsive receptor-IgG2 binding. This is suggestive of the population being studied requiring more and further research into other modifiable risk factors, such as the plaque control process and efficiency. By controlling the modifiable risk factors, it will be possible to strongly suggest the nonmodifiable risk factors (e.g. genes)to be the main contributing aspect of severe or advanced periodontal destruction, provided the finding is found to be significant.
Conclusion
Within the study constraints, FcγRIIa R/H 131 genotype was
shown to be prevalent in Malay subjects. Nonetheless, the genotypes
distribution across both groups and FcγRIIa gene polymorphisms
were not significantly associated with periodontitis. However,
the findings on this genotype may serve as a baseline data for
Malay population. It is recommended an extensive future study
with a larger sample size that will justify the current findings.
Acknowledgement and Declaration
This study was supported by Universti Sains Malaysia Research
University Grant (1001/PPSG/8012283).
References
- Brett PM, Zygogianni P, Griffiths GS, Tomaz M, Parkar M, D’Aiuto F, et al. Functional gene polymorphisms in aggressive and chronic periodontitis. J Dent Res. 2005 Dec;84(12):1149-53.
- Schenkein HA. Finding genetic risk factors for periodontal diseases: is the climb worth the view?. Periodontol 2000. 2002;30:79-90.Pubmed PMID: 12236898.
- Yoshie H, Kobayashi T, Tai H, Galicia JC. The role of genetic polymorphisms in periodontitis. Periodontol 2000. 2007 Feb;43(1):102-32.
- Loos BG, John RP, Laine ML. Identification of genetic risk factors for periodontitis and possible mechanisms of action. J Clin Periodontol. 2005;32 Suppl 6:159-79.PubmedPMID: 16128836.
- Yuan ZN, Schreurs O, Gjermo P, Helgeland K, Schenck K. Topical distribution of Fc gammaRI, Fc gammaRII and Fc gammaRIII in inflamed human gingiva. J Clin Periodontol. 1999 Jul;26(7):441-7.Pubmed PMID: 10412848.
- Hans VM, Mehta DS. Genetic polymorphism of Fcγ-receptors IIa, IIIa and IIIb in South Indian patients with generalized aggressive periodontitis. J Oral Sci. 2011 Dec;53(4):467-74.Pubmed PMID: 22167032.
- Hans VM, Mehta DS, Hans M. Association of Fc gamma-receptors IIa, IIIa, and IIIb genetic polymorphism with susceptibility to chronic periodontitis in South Indian population. Contemp Clin Dent. 2015 Sep;6(Suppl 1):S141-6.Pubmed PMID: 26604564.
- Kobayashi T, Yamamoto K, Sugita N, van der Pol WL, Yasuda K, Kaneko S, et al. The Fc gamma receptor genotype as a severity factor for chronic periodontitis in Japanese patients. J Periodontol. 2001 Oct;72(10):1324-31. Pubmed PMID: 11699473.
- Loos BG, Leppers-Van de Straat FG, Van de Winkel JG, Van der Velden U. Fcgamma receptor polymorphisms in relation to periodontitis. J Clin Periodontol. 2003 Jul;30(7):595-602.Pubmed PMID: 12834496.
- Yamamoto K, Kobayashi T, Grossi S, Ho AW, Genco RJ, Yoshie H, et al. Association of Fcgamma receptor IIa genotype with chronic periodontitis in Caucasians. J Periodontol. 2004 Apr;75(4):517-22.Pubmed PMID: 15152814.
- Yap SN, Phipps ME, Manivasagar M, Tan SY, Bosco JJ. Human Fc gamma receptor IIA (FcgammaRIIA) genotyping and association with systemic lupus erythematosus (SLE) in Chinese and Malays in Malaysia. Lupus. 1999;8(4):305-10.Pubmed PMID: 10413210.
- Nicu EA, Van der Velden U, Everts V, Van Winkelhoff AJ, Roos D, Loos BG. Hyper-reactive PMNs in FcgammaRIIa 131 H/H genotype periodontitis patients. J Clin Periodontol. 2007 Nov;34(11):938-45.Pubmed PMID: 17877745.
- Parren PW, Warmerdam PA, Boeije LC, Arts J, Westerdaal NA, Vlug A, et al. On the interaction of IgG subclasses with the low affinity Fc gamma RIIa (CD32) on human monocytes, neutrophils, and platelets. Analysis of a functional polymorphism to human IgG2. J Clin Invest. 1992 Oct;90(4):1537- 46.Pubmed PMID: 1401085.
- Wilson ME, Hamilton RG. Immunoglobulin G subclass response of localized juvenile periodontitis patients to Actinobacillus actinomycetemcomitans Y4 lipopolysaccharide. Infect Immun. 1992 May;60(5):1806-12.Pubmed PMID: 1563768.
- Laine ML, Loos BG, Crielaard W. Gene polymorphisms in chronic periodontitis. Int J Dentistry. 2010 Jan 1;2010:324719-324719.
- Silness J, Löe H. Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964 Jan 1;22:121-35.
- Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533-551.
- Carlsson LE, Santoso S, Baurichter G, Kroll H, Papenberg S, Eichler P, et al. Heparin-induced thrombocytopenia: new insights into the impact of the FcgammaRIIa-R-H131 polymorphism. Blood. 1998 Sep 1;92(5):1526-31. Pubmed PMID: 9716579.
- Osborne JM, Chacko GW, Brandt JT, Anderson CL. Ethnic variation in frequency of an allelic polymorphism of human Fc gamma RIIA determined with allele specific oligonucleotide probes. J Immunol Methods. 1994 Aug 1;173(2):207-17.Pubmed PMID: 8046255.
- Tang Y, Zhang JC, Zhang WH, Pang RY. [The association between Fc gamma receptor IIA gene polymorphism and susceptibility to chronic periodontitis in Chinese Han nationality]. Hua Xi Kou Qiang Yi Xue Za Zhi. 2004 Apr;22(2):158-61.Pubmed PMID: 15190804.
- Saremi L, Esmaeilzadeh E, Ghorashi T, Sohrabi M, Ekhlasmand Kermani M, Kadkhodazadeh M. Association of Fc gamma-receptor genes polymorphisms with chronic periodontitis and Peri-Implantitis. J Cell Biochem. 2019 Mar 19.Pubmed PMID: 30887566.
- Chung HY, Lu HC, Chen WL, Lu CT, Yang YH, Tsai CC. Gm (23) allotypes and Fcgamma receptor genotypes as risk factors for various forms of periodontitis. J Clin Periodontol. 2003 Nov;30(11):954-60.Pubmed PMID: 14761117.
- Warmerdam PA, van de Winkel JG, Vlug A, Westerdaal NA, Capel PJ. A single amino acid in the second Ig-like domain of the human Fc gamma receptor II is critical for human IgG2 binding. J Immunol. 1991 Aug 15;147(4):1338-43.Pubmed PMID: 1831223.