Assessment of Interleukin-33 Gene Variants and Protein Level in Periodontal Disease among Saudi Population
Fathy M. Elfasakhany1,2,*, Zahra Alashur3, Ebtehal H. Ali1
1 Department of Basic and Clinical Oral Sciences, Faculty of Dentistry, Umm Al Qura University, Makkah, Saudi Arabia.
2 Department of Medical Biochemistry, Faculty of Medicine, Tanta University, Tanta, Egypt.
3 Dental intern program, Faculty of Dentistry, Umm Al Qura University, Makkah, Saudi Arabia.
*Corresponding Author
Fathy M. Elfasakhany,
Department of Basic and Clinical Oral Sciences, Faculty of Dentistry, Umm Al Qura University, Makkah, Abdia,715, Saudi Arabia.
E-mail: fmfasakhany@uqu.edu.sa
Received: October 07, 2020; Accepted: October 21, 2020; Published: November 10, 2020
Citation:Fathy M. Elfasakhany, Zahra Alashur, Ebtehal H. Ali. Assessment of Interleukin-33 Gene Variants and Protein Level in Periodontal Disease among Saudi Population. Int J Dentistry Oral Sci. 2020;7(11):997-1002. doi: dx.doi.org/10.19070/2377-8075-20000198
Copyright: Fathy M. Elfasakhany©2020. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Periodontal disease is an infectious disease that affects the teeth supporting structures and finally may lead to tooth
loss.Interleukin-33 (IL-33) is a recently identified member of the IL-1 family andit was reported to havea role in immune response,
bone homeostasis and osteoclastogenesis.
Objectives: To analyze and correlatethe levels of IL-33 in the plasma and the gingival crevicular fluid(GCF) and the intronic single
nucleotide polymorphism (rs1929992) in the IL-33 genein a group of Saudi individuals with moderate to severe periodontitis and
a healthy control group.
Materials and methods: Seventy unrelated individuals with moderate to severe periodontitis and seventy healthy subjects were
included in this study. The periodontal status was assessed based onplaque index, probing depth, bleeding on probing and clinical
attachment loss. IL-33 levels were estimated in the GCF and plasmain both groups by ELISA. Peripheral blood was utilized for
preparation of genomic DNA that was used for genotyping of IL-33 A/G (rs1929992) using polymerase chain reaction followed
by restriction endonuclease digestion.
Results: IL-33 concentration was higher- but not statistically significant- in the GCF of the periodontitis subjectsin comparison
with the controls (P > 0.05) while the plasma level was the same in both groups (P > 0.05). For the IL-33 rs1929992 polymorphism,
the frequencies of the IL-33 genotypes and alleles were indifferent between the control group and theperiodontitis group
(P > 0.05).
Conclusion: The levels of plasma and GCF of IL-33 could not differentiate between subjects with periodontitis and healthy
controls. In addition, the results suggested that IL-33 rs1929992 polymorphism may not have a risk of periodontitis among the
Saudis in Makkah environ.
2.Introduction
3.Material and Methods
4.Results
5.Discussion
6.Conclusion
7.Ethical Statement
8.Acknowledgment
9.Refereces
Keywords
Gene Polymorphism; Periodontitis; Interleukin-33; Polymerase Chain Reaction; Restriction Enzyme; ELISA.
Introduction
Periodontitis is an infectious inflammatory state of the tissues
surrounding the teeth [1]. The aetiology of this complex disease
is determined by both genetic and the environmental factors (e.g.,
smoking and stress) [2, 3]. The most prominent clinical features of
the condition are development of microbial plaque, periodontal
inflammation and destruction of periodontal tissue and alveolar
bone [4]. The incidence and the severity of the disease rise with
the age and usually influences every gender evenly. Meanwhile,
the periodontal disease is noticeably detected between family
members and across different generations within the same family,
suggesting that there is a genetic susceptibility to the periodontal
disease [5, 6]. Besides, numerous studies reported the role of
gene polymorphisms of several biomolecules especially cytokines
in the host response in periodontitis, and in the progress of the
disease. Such gene polymorphisms may result in a change in the
protein structure or its expression and probably lead to alteration
in the innate and adaptive immunity and may thus be deterministic
in the outcome of the disease [7]. Cytokines are small
polypeptides that have wide range of inflammatory, hematopoetic
and immunomodulatory effects. They are produced by several
types of cells including lymphocytes, dendritic cells, monocytes,
neutrophils, fibroblasts and endothelial cells [8]. Cytokines play a valuable role in inflammatory conditionslike periodontitis and
rheumatoid arthritis. Furthermore, it has been reported that IL-1
family cytokines play key roles in inflammation. Recently, it has
been suggested that IL-33, an IL-1 family member, could share in
the development and sequence of chronic periodontitis [9]. It acts
as an alarmin, chemoattractant, and nuclear factor and has been
defined to control both innate and adaptive types of immunity
[10]. It is constitutively expressed as a nuclear factor in different
cells such as fibroblasts, endothelial cells and epithelial cells. Within
the nucleus, IL-33 behaves as an endogenous molecule that
help to maintain the transcription factor NFκB and thus lowers
the expression of genes that encode the inflammatory cytokines
thereby guaranteeing tissue homeostasis. Once becameextracellularly
upon cell damage, IL-33 works as an alarmin exhibiting
proinflammatory properties [11, 12]. IL-33 performs its action
through its ST2 receptors that are expressed in several cellslike
Th2 lymphocytes, B cells andmast cells resulting inproduction of
pro-inflammatory cytokines [10, 13, 14]. In human, excess IL-33
expressionwas detected in the gingival tissue of subjectswithchronic
periodontitis and may work as a driving factor torecruitof
B and T lymphocytes that express RANK-L [15]. However, measuring
the IL-33 in the GCF showed conflicting results in subjects
affected by chronic periodontitis [16-18]. Furthermore, the study
of IL-33 polymorphisms and their association with periodontitis
were poorly studied in different ethnic populations and to the
best of our knowledge, it is not examined before in Saudi Arabia
population. Therefore, we aimedin this study to estimate IL-33
concentrationsin the GCF and plasma of the study group and
to analyze the relationship between the IL-33 A/G (rs 1929992)
single-nucleotide polymorphism (SNP)and the susceptibility to
periodontitis among Saudis in Makkah environ.
This study included 140 subjects (70 unrelated subjects affected
with moderate to severe periodontitis and 70 healthy controls)
were recruited from the dental clinic, school of dentistry, Umm
AL Qura University, Saudi Arabia. All participants were Saudis.
Both groups were matched regarding the age and gender and had
at least 20 teeth. The inclusion criteria for selection of the control
group were the absence of both periodontal and systemic
diseases. The exclusion criteria include systemic diseases, immunodeficiency
diseases, previous orthodontic therapy, pregnancy,
lactation and smoking. The sample size was determined based on
a previous report with respect to the relationship of the IL-33
polymorphism with periodontal disease [19]. The sample size was
expanded by approximately 40% to keep up the estimates at an
optimal degree of precision (5%) against the expected impact of
reduction of sample size because of rejections and dropouts.
The periodontal condition of all individuals was assessed based
onbleeding on probing depth (PD), probing (BOP), plaque index
(PI), and clinical attachment loss (CAL) by two well trained assessors.
Individuals with BOP, PD ≥ 5 mm, CAL ≥ 3 mm and
radiographic proof of bone damage ≥ 20% were involved in thepatient
group. The updated report of the American Academy
of periodontology was used to characterize the periodontitis [20].
Two to four sites/individual were sampled to obtain a suitable volume
of GCF in healthy subjects. For subjects affected with moderate
to severe periodontitis, GCF was collected from two sites
with the highest CAL and maximum bleeding score along with
radiographic evidence of alveolar bone resorption. GCF samples
were collected using microcapillary pipettes as described before
[21]. The sample collection area was air dried and protected from
saliva contamination by isolating it withsterile cotton rolls. A
universal curette was used to remove the supragingival plaque to
avoid contamination and obstruction of the microcapillary pipette
by plaque. 10 μl volumetric microcapillary pipette (Microcapillary
pipettes, Drummond Scientific Company, Broomall, USA) was
inserted into the gingival sulcus for GCF collection. The collected
GCF was mixed with a suitable volume of phosphate buffered
saline in a new plastic tube and the dilution range was recorded to
be considered during measurement. The GCF samples contaminated
with saliva or blood were excluded and discarded. The GCF
specimens were kept at -20°C untill the assay time.
Blood specimens were obtained from all participants in K3EDTA
(tri-potassium ethylene diamine tetraacetic acid) tubes. The tubes
were centrifuged at 1200 g for 5 min and the plasma was transferred
to a new plastic tube. The white cells buffy coat was used
for extraction of DNA. All samples were kept at -20˚C until the
assay time.
IL-33 levels were measured in the GCF and plasma samples using
commercially available human IL-33 enzyme-linked immunosorbent
assay (ELISA) kit (ABCAM, Cambridge, USA). Analysis
was performed following to manufacturer’s protocol.
Peripheral blood leukocytes in the white coat of collected blood
samples were used for preparation of DNA using DNA extractionkit
(QIAamp, Qiagen, Hilden, Germany) consistent with the
instructions of the manufacturer. Extracted DNA was utilized for
PCR experiment.
IL-33 polymorphism was analyzed using the PCR-restriction fragment
length polymorphism as described before [22]. The primers
were forward 5'- GAAGTCATCATCAACTTGGAACC-3'
and reverse 5'- GGATTGGAATCCCATGGTC-3'. The PCR
program consisted of a denaturation step at 94°C for 5 min, 35
cycles: 94°C 30sec, 61 °C 30sec, 72 °C 30sec and an extensionstep
at 72°C for 5 min. The PCR fragment (217 bp) was digested with
SspI restriction enzyme and then visualized under ultraviolet light.
Genotypes pattern were: G/G gave a single band of 217 bp; A/A
gave 2 bands of 134 bp and 83 bp and AG gave 3 bands of 217
bp, 134 bp and 83 bp.
Data were assessed using SPSS version 21 (SPSS Inc., IL, USA). Continuous variables were assessed using Student’s t-test, whereas
the categorical data were assessed using Chi-squared test. Odds
ratio (OR) was calculated with 95% confidence interval (CI) and
P value > 0.05 was considered statistically significant for all analyzes.
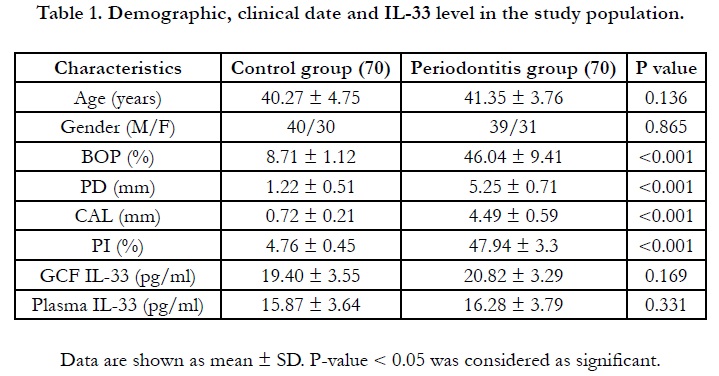
Table 1 shows that the control subjects and patients with periodontitis
were matched regarding the age and gender. The mean
age was 40.27 ± 4.75 and 41.35 ± 3.76 in controls and periodontitis
respectively (P =0.136). The gender (M/F) was 40/30 and
39/31 in controls and periodontitis respectively ((P =0.865). The
clinical data showed that the mean values of BOP, CAL, PI and
PD were significantly higher insubjects with periodontitis in comparison
with the controls (P < 0.001). This confirmed that the
matching between both groups was appropriate (Table 1).
The results of interleukin 33 level in GCF and plasma are shown
in table 1. IL-33 was detected in all GCF and plasma samples. The
mean concentration of IL-33 in GCF was19.4 ± 3.55pg/ml and
20.82 ± 3.29pg/ml in controls and periodontitis subjects respectively
(P=0.169). The mean concentration of IL-33 in plasma was
15.87 ± 3.64 pg/ml and 16.48 ± 3.79 pg/ml in the controls and
periodontitis subjects respectively (P=0.331).
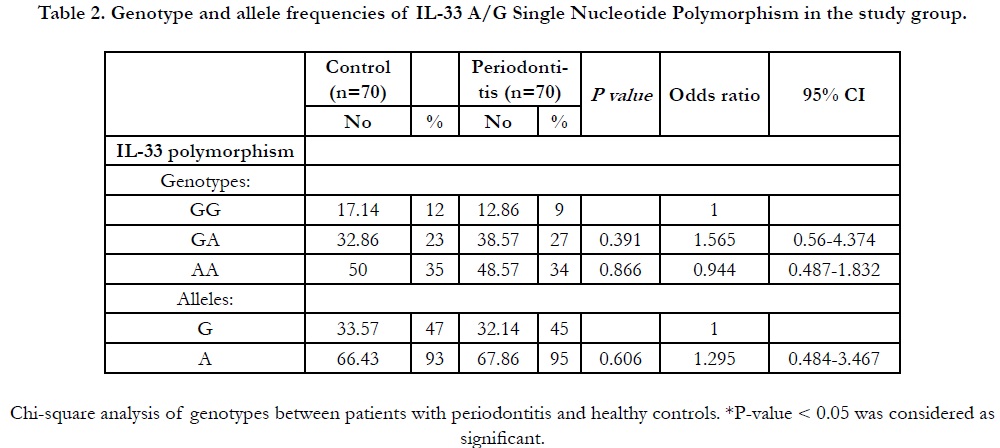
The genotypes and alleles frequencies of IL-33A/G polymorphism
in both control and periodontitis groups are shown in Table
2. The distribution of IL-33 genotypes was in Hardy-Weinberg
equilibrium in both groups. For the control subjects, the
genotypes GG, AG and AA were 17.14 %, 32.86 % and 50 % respectively
and were 12.86 %, 38.57 % and 48.57 % respectively in
the periodontitis group. The percentages of G allele were 33.57 %
and 32.14% while A allele was 66.43 % and 67.86% in the control
group and patients with periodontitis respectively. The genotype
and allele frequencies of the IL-33 showed no significant differences
between the controls and subjects with periodontitis (P >
0.05).
Table 2. Genotype and allele frequencies of IL-33 A/G Single Nucleotide Polymorphism in the study group.
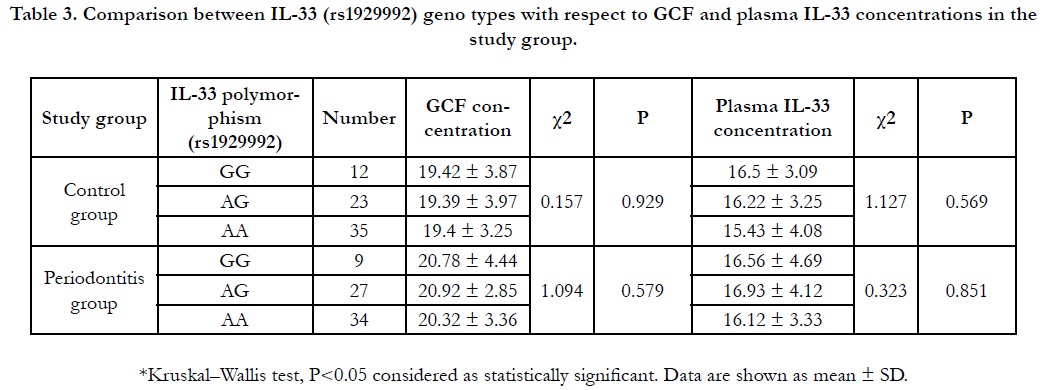
Table 3. Comparison between IL-33 (rs1929992) geno types with respect to GCF and plasma IL-33 concentrations in the study group.
The level of the GCF IL-33 in the GG, AG, AA genotypes of the
controls were 19.42 ± 3.87, 19.39 ± 3.97 and 19.4 ± 3.25 while
they were 20.78 ± 4.44, 20.92 ± 2.85 and 20.32 ± 3.36 in the periodontitis group respectively. The plasma level of IL-33 in the GG,
AG, AA genotypeswere 16.50 ± 3.09, 16.22 ± 3.25 and 15.43 ±
4.08 in the control group while they were 16.56 ± 4.69, 16.93 ±
4.12 and 16.12 ± 3.33 in the periodontitis group respectively. The
GCF IL-33 level was indifferent between different genotypes in
the controls (P = 0.929) and the periodontitis group (P = 0.579).
In addition, the plasma IL-33 level wasindifferentbetween different
genotypes in the controls (P = 0.569) and the periodontitis
group (P=0.851).
Discussion
Periodontitis is a chronic inflammation ofthe tooth surrounding
tissues that results in damage of the tooth surrounding tissue and
alveolar bone due to the interaction of the host immune response
to the pathogenic bacteria [1]. The existence of periodontopathic
bacteria in combination with elevated levels of the proinflammatory
cytokines and low levels of the inflammation inhibitory cytokines
and other factors causes the progression of periodontal
disease [23]. It is obvious that the cytokines do not work separately
but rather as a part of complex networks with diverse activities
[24]. Cytokines are released during periodontitis as a result of the
immune cells infiltrating the periodontal tissue, which eventually
causes periodontal tissue damage [25]. IL-33 is a newly identifiedmember
of IL-1 cytokine family. It can work both as a soluble
mediator and a nuclear factor. IL-33, as a cytokine, functions as anb
alarmin which isreleased upon damage of epithelial or endothelial
cells and it can target multiple types of cells thusmaking the immune
system alert to endogenous trauma likeinfection [26]. On
the other hand, it has been shown that there was a relationshipbetween
cytokinessingle nucleotide polymorphisms (SNPs) and
the periodontal disease. Such Polymorphisms canalter cytokines
production, which in turnmay lead to changes in the immune responses
and canlead tochronic inflammations [27]. As the IL-1
cytokine family is associated with chronic inflammatory and autoimmune
disorders such as rheumatoid arthritis and periodontitis
[28, 29], we aimed to estimate the IL-33 levels in the GCF and
plasma of a group of Saudi individuals with moderate to severe
periodontitis and a control group. Furthermore, since IL-33 is
a novel cytokine and its gene polymorphisms werepoorly studied
in the periodontal disease, we investigated the association of
the IL-33 A/G rs1929992 SNP andthe periodontitis in the study
group. The GCF IL-33 level washigher-but not statistically significant-
in the periodontitis subjects than the controls while the
plasma level of IL-33 was similar in both groups. For the IL-33
A/G rs1929992 polymorphism, we found no association between
IL-33polymorphism and the risk of periodontitis in the studied
Saudi subjects. The GCF and the plasma concentrations of IL-33
were measured in the periodontal disease by several investigators
but the results showed remarkable inconsistency. Ballambettu et
al (2019) could detect IL-33 in all examined samples and they
found that both GCF and plasma concentrations of IL-33 were
significantly higher in the aggressive periodontitis than chronic
periodontitis or the controls but were indifferent between chronic
periodontitis and the controls [30]. This result was consistent with
our results regarding chronic periodontitis. Buduneli et al, (2012)
found a significant lowerlevel of the IL-33in the GCF in chronic
periodontitis than subjects with healthy periodontium, whereas
the total amounts were similar in both groups butthe plasma and
salivary concentrations of IL-33 showed no difference between
the studied groups. They concluded that the IL-33 concentrations
in the GCF, saliva or plasma cannot differentiate between chronic
periodontitis and healthy individuals [31]. Their results were
consistent with our results regarding the plasma GCF level. They
stated that the episodic nature ofthe periodontal disease may be
a factor and indicated that it could be considered that the quiescent
form of periodontal tissue damage in chronic periodontitis
subjects lead to absence of significant difference between chronic
periodontitis subjects and periodontally healthy subjects in terms
of IL-33 concentrations in the biologicfluids. Furthermore, they
found that the IL-33 concentration in the GCF was significantly
lower in chronic periodontitis subjects than in control subjects.
They pointed out that the lower volume of the GCF specimens
in the periodontally healthy subjectsmay explain the significantlyhigher
IL-33 levelin the GCF in the healthy subjects than in
the chronic periodontitis. Sağlam et al, (2017) found that the total
amount of the IL-33 in the GCF was higher in chronic periodontitis
compared to the controls while the GCF IL-33 level was
lower in chronic periodontitis subjects compared to the controls.
They also found that the plasma and salivary IL-33 levels were
indifferent between chronic periodontitis and the control groups
[16]. This result is consistent with our results regarding the plasma
level of the IL-33but the GCF IL-33 concentration differs from
our results. The lower level of GCF samples in chronic periodontitis
might be not only due to the GCF volume but may depend
also on the sampling site. For this reason, we tried toavoid collection
of GCF specimens from quiescent areas, and instead, we collected
all GCF samples from the sites with the highest CAL and
maximum bleeding score together with radiographic evidence of
alveolar bone resorption. Another study done by Papathanasiou et al, (2014) measured the IL-33 in the GCF using multiplex assay
and they could not detect IL-33 in the GCF of all studied samples
from chronic periodontitis subjects and subjects with healthy
periodontium and they concludedthat there is no association between
IL-33 andthe periodontal disease and they indicated that
the presence of auto antibodies may produce non specific bindings
which can prevent the identification ofthe cytokines in the
biological specimens using multiplex assay [17].
Kurşunlu et al, (2015) measured the IL-33 and other cytokines in
the GCF in the individuals with gingivitis, chronic periodontitis,
generalized aggressive periodontitis and healthy group and they
found that the levels of IL-33 in the GCF were indifferent between
all studied groups [18]. Their results were also consistent
with our results.
Gümüş et al, (2017) showed that the salivary concentration of
IL-33 was higher inthe chronic periodontitis group even than
aggressive periodontitis while serum IL-33 level was indifferent
and they could not definitely explain their results but thought that
both diseases may have separate pathways and so, they suggested
further research [32].
Regarding IL-33 A/G rs1929992 polymorphism, it was studied
in several chronic diseases such as systemic lupus erythematosus
[33], systemic sclerosis [34], peptic ulcer [35], Behçet's disease
[36] andischemic stroke [37] but not in periodontitis according to
our knowledge. We found no association between IL-33 A/G
rs1929992 genotype or allele frequencies and the periodontitis
in Saudis in Makkah environ. To our knowledge, this is the first
report thatstudied the association betweenIL-33 A/G rs1929992
polymorphism and the periodontal disease among Saudi subjects.
However, this study is not without limitations especially the small
sample size. So, further research will be required using a large
periodontitis cohort with clinical data, other methods like evaluation
of gene expression of the IL-33 in the gingival tissues or
intervention studies are needed to understand the role of the IL-
33 in periodontal disease.
Conclusion
Our findings suggested that the GCF and the Plasma IL-33 cannot
differentiate between the subjects with healthy periodontium
andthose with periodontal disease. Also, we concluded that the
IL-33 A/G rs1929992 polymorphism may not be associated with
the risk of periodontitis among Saudis in Makkah environ, however
more genetic analysis with larger sample size and other SNPs
and haplotype analysis are required to elucidate the role of IL-33
in the pathogenesis of periodontitis.
Ethical Statement
The institutional review board of the Faculty of Dentistry, Umm
Al Qura University, Saudi Arabia approved the study. These
guidelines of the IRB follow the Saudi and International guidelines
that follows the Declaration of Helsinki in 1995 (as revised
in Fortaleza, Brazil, October 2013). Informed consents were obtained
fromall participants.
Acknowledgment
We thank Dr. Abd ElrahmanSabry for his help in the research
unit, Um Al-Qura University, Saudi Arabia. Also, we thank Prof.
AbdElaziz Y. for his help in the section of statistics.
References
- Newman MG, Takei H,Klokkevold PR, Carranza FA (2019) Newman and Carranza's clinical periodontology. 13thedn. Philadelphia; Elsevier.p. 342- 351.
- Michalowicz BS, Diehl SR, Gunsolley JC, Sparks BS, Brooks CN, Koertge TE,et al. Evidence of a substantial genetic basis for risk of adult periodontitis. J Periodontol. 2000; 71(11): 1699-707.Pubmed PMID: 11128917.
- . Borrell LN, Papapanou PN. Analytical epidemiology of periodontitis. J. Clin. Periodontol. 2005 Oct;32:132-58.
- Ohlrich EJ, Cullinan MP, Seymour GJ. The immunopathogenesis of periodontal disease. Aust Dent J. 2009 Sep;54:S2-10.
- Könönen E, Gursoy M, Gursoy UK. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J Clin Med. 2019 Jul 31;8(8):1135.Pubmed PMID: 31370168.
- Toy VE, Uslu MO. Do genetic polymorphisms affect susceptibility to periodontal disease? A literature review. Niger J Clin Pract. 2019 Apr;22(4):445- 453.Pubmed PMID: 30975946.
- Chapple IL, Bouchard P, Cagetti MG, Campus G, Carra MC, Cocco F, et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J Clin Periodontol. 2017 Mar;44(Suppl 18):S39-51.
- Callard RE, Turner MW. Cytokines and Ig switching: evolutionary divergence between mice and humans. Immunol Today. 1990 Jun;11(6):200-3. Pubmed PMID: 2191682.
- da Luz FA, Oliveira AP, Borges D, Brígido PC, Silva MJ. The physiopathological role of IL-33: new highlights in bone biology and a proposed role in periodontal disease. Mediators Inflamm. 2014;2014:342410-7.Pubmed PMID: 24692848.
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptorrelated protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005 Nov;23(5):479-90.Pubmed PMID: 16286016.
- Byers DE, Alexander-Brett J, Patel AC, Agapov E, Dang-Vu G, Jin X, et al. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J Clin Invest. 2013 Sep;123(9):3967-82.Pubmed PMID: 23945235.
- Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, et al. IL- 33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A. 2007 Jan 2;104(1):282-7. Pubmed PMID: 17185418.
- Moulin D, Donzé O, Talabot-Ayer D, Mézin F, Palmer G, Gabay C. Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine. 2007 Dec 1;40(3):216-25.Komai-Koma M, Gilchrist DS, McKenzie AN, Goodyear CS, Xu D, Liew FY. IL-33 activates B1 cells and exacerbates contact sensitivity. J Immunol. 2011 Feb 15;186(4):2584-91. Pubmed PMID: 21239718.
- Malcolm J, Awang RA, Oliver-Bell J, Butcher JP, Campbell L, Adrados Planell A, et al. IL-33 Exacerbates Periodontal Disease through Induction of RANKL. J Dent Res. 2015 Jul;94(7):968-75.Pubmed PMID: 25808546.
- Sağlam M, Köseoğlu S, Aral CA, Savran L, Pekbağrıyanık T, Çetinkaya A. Increased levels of interleukin-33 in gingival crevicular fluids of patients with chronic periodontitis. Odontology. 2017 Apr;105(2):184-190.Pubmed PMID: 27363844.
- Papathanasiou E, Teles F, Griffin T, Arguello E, Finkelman M, Hanley J, et al. Gingival crevicular fluid levels of interferon‐γ, but not interleukin‐4 or‐33 or thymic stromal lymphopoietin, are increased in inflamed sites in patients with periodontal disease. J Periodontal Res. 2014 Feb;49(1):55-61.
- Kurşunlu SF, Oztürk VÖ, Han B, Atmaca H, Emingil G. Gingival crevicular fluid interleukin-36γ (-1F8), interleukin-36γ (-1F9) and interleukin- 33 (-1F11) levels in different periodontal disease. Arch Oral Biol. 2015 Jan;60(1):77-83.Pubmed PMID: 25247780.
- Ballambettu SP, Pradeep AR, Purushottam M, Sen S. Higher interleukin-33 levels in aggressive periodontitis cases. J Indian Soc Periodontol. 2019 Sep- Oct;23(5):424-429.Pubmed PMID: 31543615.
- American Academy of Periodontology Task Force Report on the Update to the 1999 Classification of Periodontal Diseases and Conditions. J Periodontol. 2015 Jul;86(7):835-8.Pubmed PMID: 26125117.
- Kaslick RS, Chasens AI, Weinstein D, Waldman R. Ultramicromethod for the collection of gingival fluid and quantitative analysis of its sodium content. J Dent Res. 1968 Nov-Dec;47(6):1192.Pubmed PMID: 5249061.
- Fathi Maroufi N, Gholampour Matin M, Ghanbari N, Khorrami A, Amini Z, Haj Azimian S, et al. Influence of single nucleotide polymorphism in IL- 27 and IL-33 genes on breast cancer. Br J Biomed Sci. 2019 Apr;76(2):89- 91.Pubmed PMID: 30406733.
- Gemmell E, Seymour GJ. Immunoregulatory control of Th1/Th2 cytokine profiles in periodontal disease. Periodontol 2000. 2004;35:21-41.Pubmed PMID: 15107056.
- Cicchese JM, Evans S, Hult C, Joslyn LR, Wessler T, Millar JA, et al. Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol Rev. 2018 Sep;285(1):147-167.Pubmed PMID: 30129209.
- Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology 2000. 2014 Feb;64(1):57-80.
- Kurowska-Stolarska M, Hueber A, Stolarski B, McInnes IB. Interleukin-33: a novel mediator with a role in distinct disease pathologies. J Intern Med. 2011 Jan;269(1):29-35.Pubmed PMID: 21158975.
- Heidari Z, Moudi B, Mahmoudzadeh-Sagheb H. Immunomodulatory factors gene polymorphisms in chronic periodontitis: an overview. BMC Oral Health. 2019 Feb 12;19(1):29.Pubmed PMID: 30755190.
- Dinarello CA. The IL-1 family of cytokines and receptors in rheumatic diseases. Nat Rev Rheumatol. 2019 Oct;15(10):612-32.
- Shibata K. Historical aspects of studies on roles of the inflammasome in the pathogenesis of periodontal diseases. Mol Oral Microbiol. 2018 Jun;33(3):203-211.PubmedPMID: 29360244.
- Buduneli N, Özçaka Ö, Nalbantsoy A. Interleukin‐33 levels in gingival crevicular fluid, saliva, or plasma do not differentiate chronic periodontitis. J Periodontol. 2012 Mar;83(3):362-8.
- Gümüş P, Nizam N, Nalbantsoy A, Özçaka Ö, Buduneli N. Saliva, Serum Levels of Interleukin-21, -33 and Prostaglandin E2 in Patients with Generalised Aggressive or Chronic Periodontitis.Oral Health Prev Dent. 2017;15(4):385-390.
- Zhu X, Xie L, Qin H, Liang J, Yang Y, Xu J, et al. Interaction between IL-33 Gene Polymorphisms and Current Smoking with Susceptibility to Systemic Lupus Erythematosus. J Immunol Res. 2019 Mar 11;2019:1547578.Pubmed PMID: 30984790.
- Koca SS, Pehlivan Y, Kara M, Alibaz-Oner F, Oztuzcu S, Yilmaz N, et al. The IL-33 gene is related to increased susceptibility to systemic sclerosis. Rheumatol Int. 2016 Apr;36(4):579-84.Pubmed PMID: 26743213.
- Bassagh A, Jafarzadeh A, Kazemipour N, Nemati M, Aminizadeh N, Larussa T, et al. Decreased circulating interleukin-33 concentration in Helicobacter pylori-infected patients with peptic ulcer: Evaluation of its association with a cytokine gene polymorphism, gender of patients and bacterial virulence factor CagA. MicrobPathog. 2019 Nov 1;136:103708.
- . Koca SS, Kara M, Deniz F, Ozgen M, Demir CF, Ilhan N, et al. Serum IL- 33 level and IL-33 gene polymorphisms in Behçet's disease. Rheumatol Int. 2015 Mar;35(3):471-7.Pubmed PMID: 25119832.
- Guo L, Zhou X, Guo X, Zhang X, Sun Y. Association of interleukin-33 gene single nucleotide polymorphisms with ischemic stroke in north Chinese population. BMC Med Genet. 2013 Oct 9;14:109.Pubmed PMID: 24107076.