Evaluating Chitosan Effectiveness as Hemostatic Agent on Patients on Antiplatelet Therapy
Hazem Redwan1*, Muneer Harfoush1, Bassel Al Brad1, Majid A Abo Fakher2
1 Department of Oral & Maxillo facial Surgery, Faculty of Dental Medicine, Damascus University, Damascus, Syria.
2 Department of Oral medicine, Faculty of Dental Medicine, Damascus University, Damascus, Syria.
*Corresponding Author
Hazem Redwan,
Department of Oral & Maxillofacial Surgery, Faculty of Dental Medicine, Damascus University, Damascus, Syria.
Tel: +963-954-635-751
E-mail: redwanhazem@gmail.com
Received: September 19, 2020; Accepted: October 02, 2020; Published: October 06, 2020
Citation:Hazem Redwan, Muneer Harfoush, Bassel Al Brad, Majid A Abo Fakher. Evaluating Chitosan Effectiveness as Hemostatic Agent on Patients on Antiplatelet Therapy. Int J Dentistry Oral Sci. 2020;7(10):832-839. doi: dx.doi.org/10.19070/2377-8075-20000164
Copyright: Hazem Redwan©2020. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Aim: Evaluating the effect of chitosan on hemostasis has taken a plethora of outcomes in the current dental medicine literature.
Nonetheless, this split-mouth randomized controlled trial comes to investigate the correlation between two groups
- patients on antiplatelet therapy, aspirin - in terms of bleeding time, as the only variable.
Materials and Methods: 40 patients were included in this study, who had two opposite extraction sockets on opposite sides
of the jaws. They were divided into the control and experimental groups; they both received gauze dental dressing for the
former group and chitosan for the latter. Hemostasis was measured via a new method that has not been utilized before in the
literature. The bleeding event was recorded and data were analyzed using SPSS and MS Excel.
Results: It has been found that a low correlative coefficient value, plummeting to only 0.136, existed for the 80-tooth samples
in both the control and experimental groups. This has been ascribed to the nature of the hemostatic mechanism of chitosan,
as a renowned marine biopolymer in dental practices.
Conclusion: INR values seemed unlikely to support the existent claims on chitosan effect, leaving a venue to interrogate the
explanation that validates the electric aggregation link between chitosan and red blood cells to reach the hemostasis event.
Clinical Significance: Measuring the bleeding time, besides, was done in an unconventional process to monitor the bleeding
intervals. Further research is suggested to evaluate this present study, taking into account the surrounding factors that interfere
in the chitosan-enhanced hemostasis process, being thermal or mechanical.
2.Introduction
3.Materials and Methods
4.Results
5.Discussion
6.Conclusion
7.References
Keywords
Chitosan; RBCs; Aggregation; Hemostasis; Bleeding Time; Antiplatelet Therapy.
Introduction
Tooth loss is considered the widest problem amongst health issues;
likewise, exodontia cases have been witnessed as the most
pervasive procedure in clinical settings [1]. Nonetheless, tooth extraction
in both of its types, simple and surgical, is surrounded by
a plethora of dangers, such as bleeding, hematoma and infection
[2]. Building on the aforementioned statement, it is prerogative at
this stage to address the issue that lessens the adverse effects of
these three factors, hemostasis.
Post-extraction bleeding is seen as a prime hindrance in the context
of maxillofacial surgeries; further, any modification of antiplatelet
medication has been found to lead to excessive proneness
to cardio infarction and cerebral clotting. Therefore, it is wise to
avoid changing those drugs whenever possible [3], as bleeding
must be held at a focal point of consideration when coagulation
is seen at critical levels. Hemostasis, thus, comes to the façade of
solutions.
Hemostasis agents vary depending on cost, effectiveness and suitability.
The hemostatic agent, to be optimal, must meet the following
criteria; it should be safe, tolerable to be applied, suppressing
to microorganisms, sterilized to be utilized for one before being
disposed of [4]. On the contrary, aspirin has prominent properties
to exacerbate the hostile setting against any hemostatic agent.
Actually, anticoagulants are recognized to host such underpinnings
on the mechanism of hemostatic agents where severe postextraction
bleeding is difficult to be stopped by regular gauze [5].
Recently, some marine crustacean products have been theorized to possess properties represented by biocompatibility and bacteria-
resistant features. Therefore, the rationale behind this study
has been to evaluate the effectiveness of one of the mostly utilized
agents to contribute to clotting the bleeding resultant from
exodontia, chitosan. Chitosan was first explored by Dr. Rouget
after treating the chitin with the soluble potassium hydroxide.
Chitosan is manufacture via dry-iced chitin, which is derived from
the prawn’s shell [6, 7]. To decide on the best hemostatic agent for
those on antiplatelet therapy, an urgent need is seen to consider
chitosan as it is cheap to obtain, abundant in nature, biodegradable,
non-toxic and antimicrobial.
On top of all its properties, chitosan is extensively used via the
military sector in the United States due to its proven advantages as
a premium hemostatic agent [6]. It, moreover, possesses a highly
positive electrical charge that prompts the surrounding cells to
coagulate in a specific point of application. In this regards, it is
imperative to recite the main four phases of any wound healing.
The first one is hemostasis, seconded by inflammation. The third
stage is proliferation of the tissue, and finally comes remodeling.
Some studies have elaborated the important properties of chitosan
in scaffolding each of these stages [8]. This can be explained
throughout the fact the certain cells responsible for wound healing
is directly influenced via the chitosan mechanism. Additionally,
wound sites are highly rich with collagen after the application
of chitosan; this is represented by the thick availability of white
blood cells in these sites [9].
This is a randomized controlled trial (RCT) following the splitmouth
model; it is also double-blinded for two reasons. First, the
second clinician who recorded the clotting times was not knowledgeable
of the type of dressing applied to the extraction sockets
in each patient, be it chitosan or normal gauze. Second, the patient
was not informed about which side of the mandible/maxilla
included which dressing to avoid any bias regarding reaching the
clotting stage with the help of biting; this criterion per se was not
found in any of the articles used to cover our topic beforehand, to
the humble knowledge of this paper and its researchers. Nonetheless,
the RCT included two groups, the experimental and the control
ones. The experimental group – half of the extraction sockets
in all patients - admitted the chitosan as the hemostatic medium,
where the second group received only normal gauze dressing. The
side receiving chitosan was selected randomly via tossing a coin to
choose which side to admit the chitosan; henceforth, each patient
receive two type of dressing after extraction: chitosan and normal
gauze.
The study included 40 patients whose extraction sockets were distributed
evenly (40 against 40) and randomly onto the two study
groups; their names are referred to in the form of numbers to
meet the requirements of the Ethics and Manners Committee.
Each patient has one extraction socket on both the right and left
sides of the jaws, so these sockets were opposite each other to
apply the chitosan dressing on one and the normal gauze on the
other. Those patients were admitted at the Oral and Maxillofacial
Surgery Hospital at the Faculty of Dental Medicine, Damascus
University; their ages range 40-70 years. Besides, a written consent was obtained from each patient to enroll in this study, and a
study registration permit was obtained from the Ethics and Manners
Committee to initiate the study prior to obtaining the patient
consent.
The patient who were included in this study fulfilled the following criteria. Each patient:
• ought to be one on oral antiplatelet therapy,
• had two counterpart teeth one both sides of the dental arches which needed extraction
• was on good oral hygiene, caring of their teeth
• aged 40 to 70 years
On the other hand, patient exclusion criteria included the following.
The patient was not admitted to this research if he/she:
• was alcoholic or smoker
• had allergy against marine food
• was on anticoagulants affecting the clotting flow
Chitosan, manufactured under the trademark Axiostat (Gujarat,
India), was used in the form of a packaged sponge for each patient.
In addition, clinical diagnostic tools were used along with
the anesthesia equipment. Final, the simple extraction tools were
the final of the needed materials.
Upon acquainting each patient with the form paper designed
for this research, their written consent was obtained along with
their commitment to complete all treatment stages including the
follow-up dates. Moreover, the personal information was taken
for each patient accompanied by their medical and dental history.
Later, each patient was clinically and radiographically diagnosed
to make sure that the case needs extraction.
After this initial stage, laboratory analyses were sought to confirm
the suitability of each patient to accept the treatment. Those
blood tests included CBC, Bleeding Time, PT, and INR. After
insuring that each patient met the inclusion criteria and was ready
to receive the treatment, each extraction site from the two in each
patient was chosen randomly via flipping a coin (head/tails). Further,
the patient was not informed of which extraction socket
received which dental dressing.
Anesthesia was utilized via applying lidocaine 2% with adrenaline
1/80000; the periodontium ligaments were cut, and all teeth received
simple extraction, with the minimal tissue trauma. Then,
both types of dental dressing were applied to the extraction socket
in a way that the dressing was levelled with the apex of crustal
bone, insuring that the excessive ridges of both of the dental
dressings were cut so that the dressing comes on one surface with
the extraction socket.
The bleeding index was evaluated according to the next route.
First, the hemostasis time was recorded by another dentist who
had no clue regarding the type of dental dressing that had been applied. Timing was recorded according to timing intervals
grouped in 30-second segments. The second dentist/clinician
recorded the bleeding time using a stopwatch, deciding on the
clotting time as follows. The two extraction sites were observed
every 30 seconds; if clotting achieved, in the 37th second for example
- this cannot be determined unless the borders of interval
is reached - the interval was recorded as clotting happened in the
second one. Therefore, clotting in this case was recorded to be
achieved under the borders of 1 min. The second dentist who
recorded the clotting time observed bleeding every 30 seconds;
to the humblest knowledge of this paper and its researchers, this
method has never been employed before; amongst a considerable
number of articles on achieving the clotting time, Azad et al.
postulated the concept [10].
Nevertheless, at the end of each 30-second interval, the blood
extending beyond the extraction socket borders, i.e. to the gingival
ridges, was dried by the second dentist/clinician using sterilized
gauze. When the next interval check comes and clotting has been
achieved, bleeding time is recorded in this very next interval. At
this stage of the research paper, it is vital to mention that bleeding
time means the time at which bleeding, extending beyond the
extraction socket, stops [11].
Each patient, later, was given written and spoken instructions as
to exclude worse scenarios resultant from ignorance of preserving
the wound site. This was escorted by confirming attendance
of the follow-up schedule. Still, the patient stayed in the hospital
after extraction for one hour to monitor and evaluate any secondary
bleeding contraindications [12]; this step per se was traced via
phone calls in none was observed in the hospital but appeared
after the span of one hour.
Seventy-two hours later, the patient was admitted to the follow-up
session, when chitosan is most probably completely biodegradable
in the living tissues. This was performed so that remaining
chitosan was removed once traces of excessive remaining were
observed in the socket; the remaining chitosan was treated with
saline, as it is soluble then [13].
Results
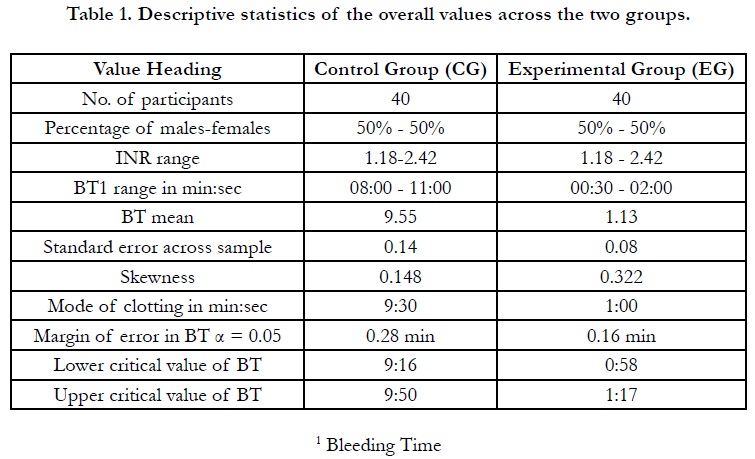
To begin this section, a number of findings must be initially stated.
First, the bleeding time will be interchangeable in meaning
with clotting time. Moreover, the values recorded by the second
clinician about the bleeding time (BT) ranged in the control group
between 8 to 11 minutes. Their counterpart values plummeted
to 0.5 minutes, i.e. 30 seconds, to 2 minutes in the experimental
group, which were subjected to gauze dental dressing. Nevertheless,
the statistical values are sometimes recorded in the format
of percentages not in the integer values of seconds. For example,
0.16 min will be read to equal approximately 10 seconds.
Moreover, data were analyzed utilizing a number of tests with the
help of the statistical package of IBM, SPSS v26, along with Microsoft
Office Excel 2019. Descriptive data were also obtained for
both of the groups; the most important of which are included on
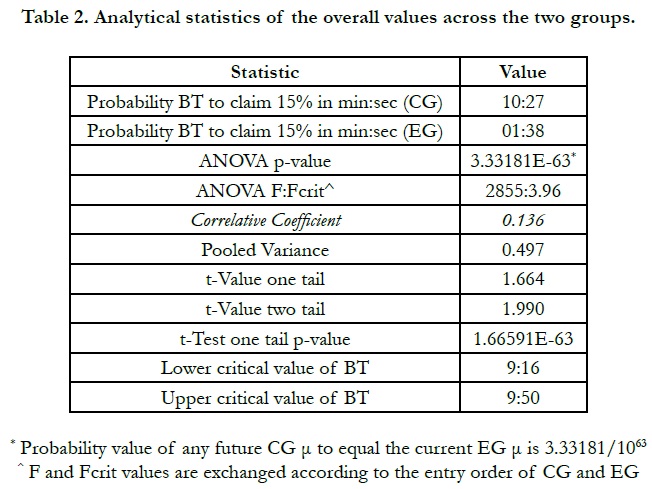
Table 1. Some predictive statistics for the validity and reliability of
this research are also found on Table 2 to promulgate future probabilities
for the coming researchers if the topic is sought to be
repeated by any researcher. Still, there are some findings that have
never been mentioned in the reviewed previous studies; these
findings need to be further investigated by future researchers.
Finally, in this introductory segment of this section, Results, it is appropriate to mention that the following criteria were applied to test the results:
1. ANOVA
2. T-Test
3. Probability principles
4. Z-Test (though NOT included on any table, since its p-values endorses those in the t-Test and ANOVA. To avoid repeating the values, the z-Test values were intentionally omitted)
BT in the experimental group, as is demonstrated on Table 1, showed values to score between a half to two minutes with the help of chitosan application in the post-extraction socket. Further, the table per se highlights the number of participants, 40 patients, distributed evenly according to both of gender, 50% for each.
It can be reiterated from Table 1 that the standard error is decreased in the EG to yield consistent data; this is easily contrasted to the 0.14 in the CG where the clotting time claimed more widely scattered data. A point that deserves focused discussion in the next section of this article.
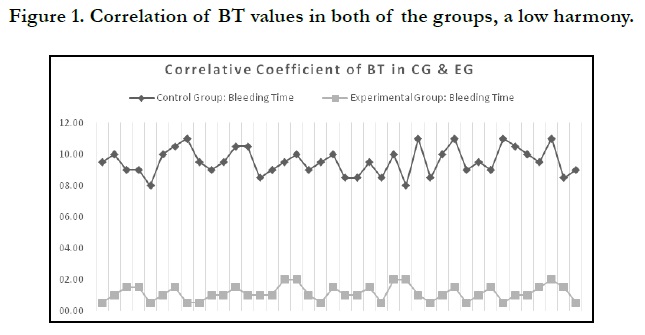
Another important finding, which is claimed to be novel in the research on chitosan as a sufficient hemostatic agent, is the lack of correlation between the clotting times in the CG and their counterpart values in the EG. This is to say, if the patient’s bleeding time were 10:30 min, for instance, and their BT in the socket that received chitosan post-extractive dental dressing were 01:00, for instance, it would not be necessary that a CG patient with 09:00 BT should achieve a clotting time to be 01:00 or less. Henceforth, the correlative coefficient between the CG and EG is statistically low, as is displayed on Table 1 and illustrated in Figure 1. The confidence level at which the data were analyzed is Alpha 0.05, α = 0.05. This was chosen to come in tandem with other previous studies.
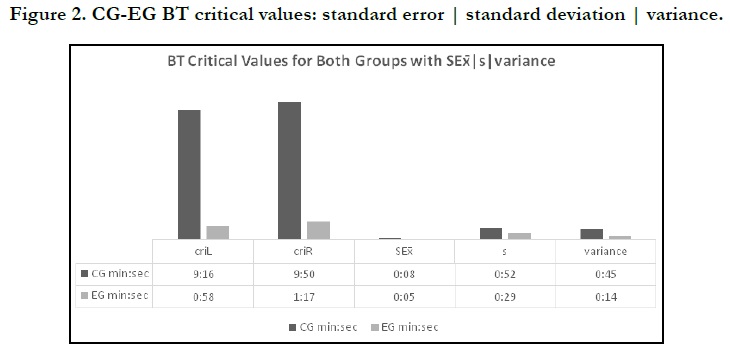
Nonetheless, another finding that is striking to shed light on is the critical value of BT. The lower critical value will be coded as criL, whereas the upper critical value is coded criR that necessarily falls on the furthest point to the right of Gaussian Curve in a normal hypothetical distribution is plotted used our current data. This is to say, any future sample - building of the CG current sample - will probably achieve clotting post-extractive time at the interval values of criL = 09:16 and criR = 09:50. These two aforementioned times will decrease to meet the EG chitosan-enhanced hemostasis at criL = 00:58 and criR = 01:17. The former discrete values are not but an emphasis of the efficacy of such antibleeding agent; the fact had better be represented in the following figure to dichotomize the variance endpoints, Figure 2.
On another though, the INR does not seem to play a decisive factor in studies such as this once, namely RCT undergoing the split-mouth model. This statement depends on the factor that this static variable abides in each of the patients; thus, the ensuing BT values will necessarily connect to the same INR. Nevertheless, its presences within the range of 1.18-2.42 calls forth additional concepts to be postulated at in the coming section, Discussion. The INR values invite more points to be raised regarding patients on antiplatelet therapy, the aspirin in our case.
Discussion
Chitosan as a biopolymer being able to be resorbed within body
tissues has been found in this study to procure a solid ground for
the damaged wound cells to regenerate and grow in a significant
way to help stop bleeding [14]. It helps reduce inflammation after
surgery; however, the inflammation index was ignored in our current
investigation, whereas it was highlighted by another study on
a sample subgroup [15]. No parameters were detected in our case
to employ such criteria to test chitosan; this inflammation link is
left for the evaluation of future studies.
It is well established that any hematological treatment is seen vital
for those on anticoagulation medicines; the properties of blood
flow call for sufficient remedy. Where it is commenced via aspirin
pre-operatively, it is seconded by chitosan after surgical opening
of the skin to inhibit such flow [16]; however, other haemophilia
contraindications related to thermos-factors of the setting were
controlled within the temperature of the operation room. These
factors may affect BT; this was not monitored by Efeoğlu et al.
in their study, while it was here. Moreover, since the bleeding and
thermo-related factors occur within the borders of the oral cavity,
any gastrointestinal complication caused by the antiplatelet
medicine is unlikely to shed direct effects of the process of clotting;
therefore, this was ignored in our study [17]. The chitosanenhanced
BT mean is recorded in the EG to be 1.13 min and 9.55
min in the CG that is other side of each split-mouth in our study;
these BTs need further comparison with the 41-patient sample in
the study by Madan et al.; their pre-operative BT μ equaled 2.86
min.
Recording and photographing the post-operative surgery site has
predominantly been an obsession to the mind of the researchers
in our study. This phenomenon can be negotiable on different
grounds. For instance, Sarkar et al. perceived tracing the exodontic socket to be photographed post-operatively in one day and
three days; confer Figure 2 in Sarkar et al.’s study [18]. We found
no need to trace graphically the extraction socket after the patient
leaves the hospital because a host of factors can never be
controlled then which affect the proliferation of wound tissues.
Henceforward, the images were taken within the venue/span of
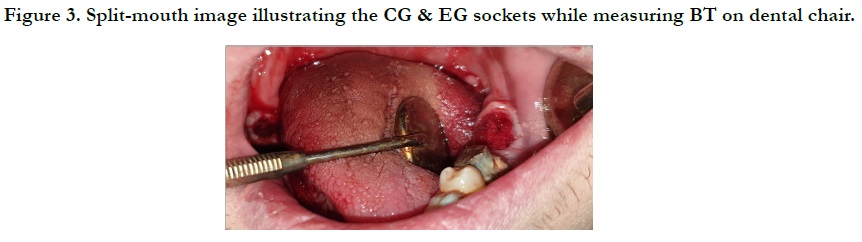
the direct surgical interference as is illustrated in Figure 3.
Bleeding time does not relate to thermal external factors, on the first though, in the majority of the contacted references, for instance Jimson et al.’s study [19]. Nonetheless, temperature and liquids seem to play a decisive factor in the workability of chitosan. This is built upon the findings of Liu et al. who theorize that the natural cross-linker, genipin, is sufficient enough to fix the amino groups on chitosan [20]. What is more, in other literature studies on chitosan, absorbable acids like vitamin C are found to help activate the chitosan taken in the stomach and intestine [21]. In all, internal and external factors that may interfere in the chitosan mechanical process are suggested by our study to be monitored to the maximum limits. Unfortunately, one of the failures that we unintentionally committed in this humble research is our ignorance of recording the thermal external factor at the application of chitosan on the experimental extraction sites.
Cytokine, regarding wound healing, has been at a focal point when conducting this investigation. It activates cell proliferation [22]. Seeking a proper method to enhance such bridging can be attributed to chitosan with the BT in EG not exceeding the borders of 02:00 min. Future research is invited to instigate a comparison between chitosan and other less costly extensively abundant practices in utilizing the tranexamic acid mouthwash onto those on antithrombotic medicine. Besides, we call to associate the application of chitosan only on patients with high INR levels, unlike Cañigral et al. who concluded that moderate levels of INR hindered concluding results on BT [23]. In our case, the INR range of 1.18-2.42 held optimum value in testing chitosan; prior to such generalization, we did contemplate the INR-PT link to be operational here.
Our patients here take aspirin on regular basis; CG BT does indicate a need to stop the anticoagulant due to excessive blood loss when extracting the tooth. Being major, the BT is statistically significant to consider specifically when it fluctuates between 08:00- 11:00 min, only to contrast the finding of Nooh who did not postulate over the significance of blood loss post-operatively [24] opposing Brennan et al. whose study outcome was seconded by our CG BT [25]. They reiterated the withdrawal elevation to be affecting blood pressure; it can be easily considered hazardous upon withdrawal of beta-blocker regimens.
Coagulopathy leads to inflammation. This phenomenon was at its low levels when monitoring the experimental group subjected to chitosan. To begin, the mechanism of chitosan stems from its highly-positive charge as a marine compound, which aggregates the negatively charged blood cells at the locus of operation site being bleeding [26]; this leads to blood viscosity that in turn contributes to formulating the required clot. Here, it is advisable by this paper to investigate severer environment against chitosan by considering enrolling a sample of patients on antiplatelet therapy taking clopidogrel not aspirin. The former was found to create harsher environment regarding atrial fibrillation and blood fluidity. Still, the mechanism of chitosan does not yield this beneficial stage of clotting; chitosan extends to fight bacteria. The complications against the treated tissues in the EG were observed to be fewer than the CG; this may be ascribed to the anti-bacterial properties of chitosan [27]. These properties were further scrutinized in vitro, where chitosan was found to increase the inner and outer membrane permeability, leading to impair of the bacterial cell membranes.
Oozing was observed in some CG surgical sites post-operatively in two days, but was not controlled or monitored as other studies. However, BT was the focal point in our study, which ranged in the CG between 08:00 to 11:00 min only to oppose Willem et al. whose anticoagulant-taking patients recorded BT to be fewer than 30 min [28]. This can be ascribed to the abnormal INR levels in that study. In the background of this present study, there was a need to utilize bio-compound fully compatible with the connective tissues and achieving regenerative healing, namely chitosan. The application of this compound on such a small-scaled sample allowed us to scrutinize its properties, unlike wide-scaled studies such as Lu et al. who included 1669 CG extraction sites [29]. Likewise, the chitosan healing properties in our study were traced accurately and individually, as recommended earlier in the literature. We worked on minute detailing of the bleeding time via adopting measuring steps to record the bleeding event, which has not been mentioned in the literature of using chitosan as a dental postoperative hemostatic, utilizing chitosan in its sponge form; this is one of the available forms in the market these days.
Depending on our results, it was, furthermore, confirmed that chitosan yielded low-to-zero periodontal complications. Chitosan is well known to inhibit the growth of porphyromonas gingivalis and aggregatibacter actinomycetemcomitans [30]. Chitosan did reduce the time of hemostasis; it could, actually, be used in extreme cases when the patient suffers coronary angiography [31], let alone our normal bleeding cases where the expectation should be high from its application within the sample’s individuals. Our sample, in it’s both the CG and EG was seen as a whole collective outcome regarding the results. First, it is recommended for the future researchers that Friedman’s Test be employed to compare the BT values within the group itself, not only across the groups, as is our case. Second, the application of chitosan prevented, within our sample, the invasive visits after dental extraction, specifically for those on antiplatelet medication. In all, the extraction in our cases for both of the groups took the form of gentle luxation prior to extracting the teeth. This has been delivered to eradicate, or rather minimize, the availability of tooth residues that interfere in the process of tissue regeneration/proliferation, where the marine product chitosan is procured a clear ground to work as a scaffold to allow such cell regeneration. However, we cannot recommend enhancing the mechanism of chitosan with other materials, such as Dexamethasone, for the reason of the sufficiency of such a sea-originating biopolymer to fulfill hemostasis alone [32].
On the one hand, chitosan forms a smooth scaffold network, integrating with the bio-structure in an adequate cross-linking. One the other hand, haemophilia underpins a persistent pressure in the world of surgery through its three existing stages: mild, moderate, and severe [33]. During impaired bleeding, chitosan works on the electrically charged cells as explained earlier, unlike other coagulants that stop the blood flow. In lieu of establishing a valid harmony between the counterpart values of our CG BT and EG BT, we depended on the basic features of chitosan to gravitate/ aggregate the red blood cells (RBCs) when applied. Therefore, the values of BT in the CG are statistically inappropriate to affect or relate to their counterparts in the EG in our split-mouth RCT. However, what lacks in the literature and in our study, also, is a call for employing the complete blood count (CBC) prior to initiating a surgical interference upon patient on antiplatelet therapy; we claim that chitosan is best be seen in this context, i.e. its connectivity to the cell charge not the cell fluidity path in open dental wounds. The magnitude of bleeding may not formulate an ongoing obstacle in the way of dentist, where there is a call to reduce the INR levels in patients to 1.5 [34]. A number of studies that are proponents of partial discontinuation of anticoagulants focus mainly on blood flow when bleeding, with the minimal or non-existent fact of the electrical mechanism of chitosan. It is worthwhile mentioning that some manage this idea of bleeding via inserting oxidized regenerated cellulose [35] ignoring the aforementioned mechanism of chitosan.
In the final stage, some statistical findings are suggested to be discussed. First, the discontinuation of taking aspirin was not a choice to be supported throughout this present study. The chitosan- enhanced hemostasis values proved considerably more proficient than what was stated by Fernandez and Lee [36]. When small wounds are addressed, the formula of chitosan is recommended for hemorrhage; however, the sponge form might prove more effective to reshape in many forms, so that it fits the postextraction socket, leaving the minimal over-fitting edges. Second, the general acceptable INR values for surgeries are theorized to range between 2.5 and 3.5 [37]; ours ranged 1.18-2.42, questioning the validity of chitosan application at such theorized levels mentioned by the very previous study. Third, the muco-adhesive properties of chitosan have been theorized to block the flow of blood via allowing the blood to interact with its structure, formulating an adequate clot [38]. The electro-aggregation of chitosan is postulated - by this present study - to play a major role in paving the way to scaffold the cloth, rather than interacting with the blood. This may explain the low correlative coefficient values between the CG and EG in this current study. Nonetheless, the mechanical adhesion of chitosan proved effective when forming a smooth mesh in the EG post-extraction sites. Having stated that, the attraction of RBCs can be seen as the key to achieve satisfactory results when reaching the hemostatic stage. As aspirin functions throughout inhibiting irreversibly the platelet function and cyclooxygenase through a selective acetylation of human COX-1, lasting for the life of the platelet [39], it is unlikely that the flow of the platelets will be immediately affected. Rather, the charged build of the cell perhaps plays a decisive role in the overall effect of this proficient marine product, chitosan. In all, if surgery is meant to be performed to patients taking antiplatelet therapy, the anticoagulants are not probably discontinued [40], inviting more adequate research on chitosan and its surrounding factors that necessarily interfere in its mechanism. Some thermal and mechanical factors have been theorized in this section, Discussion, to affect chitosan application, but more measures - also mentioned in the venue of this section - need to be considered for future studies.
References
- Nathwani S, Martin K. Exodontia in dual antiplatelet therapy: the evidence. Br Dent J. 2016 Mar 11;220(5):235-8. Pubmed PMID: 26964594.
- Sharma S, Kale TP, Balihallimath LJ, Motimath A. Evaluating Effectiveness of Axiostat Hemostatic Material in achieving Hemostasis and Healing of Extraction Wounds in Patients on Oral Antiplatelet Drugs. J Contemp Dent Pract. 2017 Sep 1;18(9):802-806. Pubmed PMID: 28874645.
- Salam S, Yusuf H, Milosevic A. Bleeding after dental extractions in patients taking warfarin. Br J Oral Maxillofac Surg. 2007 Sep;45(6):463-6. Pubmed PMID: 17250937.
- Amer MZ, Mourad SI, Salem AS, Abdelfadil E. Correlation between International Normalized Ratio values and sufficiency of two different local hemostatic measures in anticoagulated patients. Eur J Dent. 2014 Oct;8(4):475-480. Pubmed PMID: 25512727.
- Pozza M, Millner RW. Celox (chitosan) for haemostasis in massive traumatic bleeding: experience in Afghanistan. Eur J Emerg Med. 2011 Feb;18(1):31- 3. Pubmed PMID: 20461007.
- Malmquist JP, Clemens SC, Oien HJ, Wilson SL. Hemostasis of oral surgery wounds with the HemCon Dental Dressing. J Oral Maxillofac Surg. 2008 Jun;66(6):1177-83. Pubmed PMID: 18486782.
- Matica MA, Aachmann FL, Tøndervik A, Sletta H, Ostafe V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int J Mol Sci. 2019 Nov 24;20(23):5889. Pubmed PMID: 31771245.
- Cunha-Reis C, TuzlaKoglu K, Baas E, Yang Y, El Haj A, Reis RL. Influence of porosity and fibre diameter on the degradation of chitosan fibre-mesh scaffolds and cell adhesion. J Mater Sci Mater Med. 2007 Feb;18(2):195- 200. Pubmed PMID: 17323150.
- Behrens AM, Sikorski MJ, Kofinas P. Hemostatic strategies for traumatic and surgical bleeding. J Biomed Mater Res A. 2014 Nov;102(11):4182-94. Pubmed PMID: 24307256.
- Azad AK, Sermsintham N, Chandrkrachang S, Stevens WF. Chitosan membrane as a wound-healing dressing: characterization and clinical application. J Biomed Mater Res B ApplBiomater. 2004 May 15;69(2):216-22. Pubmed PMID: 15116411.
- Kumar KR, Kumar J, Sarvagna J, Gadde P, Chikkaboriah S. Hemostasis and Post-operative Care of Oral Surgical Wounds by Hemcon Dental Dressing in Patients on Oral Anticoagulant Therapy: A Split Mouth Randomized Controlled Clinical Trial. J ClinDiagn Res. 2016 Sep;10(9):ZC37-ZC40. Pubmed PMID: 27790577.
- Mingarro-de-León A, Chaveli-López B, Gavaldá-Esteve C. Dental management of patients receiving anticoagulant and/or antiplatelet treatment. J ClinExp Dent. 2014 Apr 1;6(2):e155-61. Pubmed PMID: 24790716.
- Kong M, Chen XG, Xing K, Park HJ. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol. 2010 Nov 15;144(1):51-63. Pubmed PMID: 20951455.
- Azargoon H, Williams BJ, Solomon ES, Kessler HP, He J, Spears R. Assessment of hemostatic efficacy and osseous wound healing using HemCon dental dressing. J Endod. 2011 Jun;37(6):807-11. Pubmed PMID: 21787494.
- Doganay O, Atalay B, Karadag E, Aga U, Tugrul M. Bleeding frequency of patients taking ticagrelor, aspirin, clopidogrel, and dual antiplatelet therapy after tooth extraction and minor oral surgery. J Am Dent Assoc. 2018 Feb;149(2):132-138. PubmedPMID: 29389336.
- Efeoğlu C, SipahiÇalış A, Karasu Z, Koca H, Boyacıoğlu H. Prospective randomized single-blind study of post-operative bleeding after minor oral surgery in patients with cirrhosis. Turk J Gastroenterol. 2019 Feb;30(2):171- 176. PubmedPMID: 30457557.
- Madan GA, Madan SG, Madan G, Madan AD. Minor oral surgery without stopping daily low-dose aspirin therapy: a study of 51 patients. J Oral Maxillofac Surg. 2005 Sep;63(9):1262-5. PubmedPMID: 16122588.
- Sarkar S, Prashanth NT, Shobha ES, Rangan V, Nikhila G. Efficacy of Platelet Rich Fibrin versus chitosan as a hemostatic agent following dental extraction in patients on antiplatelet therapy. J Oral BiolCraniofac Res. 2019 Oct-Dec;9(4):336-339. PubmedPMID: 31467833.
- Jimson S, Amaldhas J, Jimson S, Kannan I, Parthiban J. Assessment of bleeding during minor oral surgical procedures and extraction in patients on anticoagulant therapy. J Pharm Bioallied Sci. 2015 Apr;7(Suppl 1):S134-7. PubmedPMID: 26015691.
- Liu BS, Yao CH, Fang SS. Evaluation of a non-woven fabric coated with a chitosan bi-layer composite for wound dressing. MacromolBiosci. 2008 May 13;8(5):432-40.Pubmed PMID: 18273834.
- Cicciù M, Fiorillo L, Cervino G. Chitosan Use in Dentistry: A Systematic Review of Recent Clinical Studies. Mar Drugs. 2019 Jul 17;17(7):417.Pubmed PMID: 31319609.
- Dohan DM, Choukroun J, Diss A, DohanSL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral RadiolEndod. 2006 Mar;101(3):e45-50.Pubmed PMID: 16504850.
- Cañigral A, Silvestre FJ, Cañigral G, Alós M, Garcia-Herraiz A, Plaza A. Evaluation of bleeding risk and measurement methods in dental patients. Med Oral Patol Oral CirBucal. 2010 Nov 1;15(6):e863-8.Pubmed PMID: 20711153.
- Nooh N. The effect of aspirin on bleeding after extraction of teeth. Saudi Dent J. 2009 Jul;21(2):57-61. PubmedPMID: 23960460.
- Brennan Y, Gu Y, Schifter M, Crowther H, Favaloro EJ, Curnow J. Dental extractions on direct oral anticoagulants vs. warfarin: The DENTST study. Res PractThrombHaemost. 2020 Feb 11;4(2):278-284. Pubmed PMID: 32110759.
- Aguilar A, Zein N, Harmouch E, Hafdi B, Bornert F, Offner D, et al. Application of chitosan in bone and dental engineering. Molecules.2019 Aug 19;24(16):3009. PubmedPMID: 31431001.
- Kale TP, Singh AK, Kotrashetti SM, Kapoor A. Effectiveness of Hemcon Dental Dressing versus Conventional Method of Haemostasis in 40 Patients on Oral Antiplatelet Drugs. Sultan QaboosUniv Med J. 2012 Aug;12(3):330-5. PubmedPMID: 22912926.
- Schreuder WH, Peacock ZS. Antiplatelet therapy and exodontia. J Am Dent Assoc. 2015 Nov;146(11):851-6. Pubmed PMID: 26514891.
- Lu SY, Tsai CY, Lin LH, Lu SN. Dental extraction without stopping single or dual antiplatelet therapy: results of a retrospective cohort study. Int J Oral Maxillofac Surg. 2016 Oct;45(10):1293-8. PubmedPMID: 26972159.
- Wohlfahrt JC, Evensen BJ, Zeza B, Jansson H, Pilloni A, Roos-Jansåker AM, et al. A novel non-surgical method for mild peri-implantitis – A multicenter consecutive case series. Int J Implant Dent. 2017 Dec;3(1):38. Pubmed PMID: 28776288.
- Rodriguez-Merchan EC. Local fibrin glue and chitosan-based dressings in haemophilia surgery. Blood Coagul Fibrinolysis. 2012 Sep;23(6):473-6. PubmedPMID: 22688558.
- Chen CY, Chung YC. Antibacterial effect of water-soluble chitosan on representative dental pathogens Streptococcus mutans and Lactobacilli brevis. J Appl Oral Sci. 2012 Nov-Dec;20(6):620-7. PubmedPMID: 23329243.
- Liras A, Romeu L. Dental management of patients with haemophilia in the era of recombinant treatments: increased efficacy and decreased clinical risk. BMJ Case Rep. 2019 Apr 8;12(4):e227974. Pubmed PMID: 30962210.
- Wahl MJ. The mythology of anticoagulation therapy interruption for dental surgery. J Am Dent Assoc. 2018 Jan;149(1):e1-e10. PubmedPMID: 29304913.
- Bajkin BV, Vujkov SB, Milekic BR, Vuckovic BA. Risk factors for bleeding after oral surgery in patients who continued using oral anticoagulant therapy. J Am Dent Assoc. 2015 Jun;146(6):375-81.Pubmed PMID: 26025824.
- Fernandez RS, Lee A. Effects of methods used to achieve hemostasis on radial artery occlusion following percutaneous coronary procedures: a systematic review. JBI Database System Rev Implement Rep. 2017 Mar;15(3):738-764. Pubmed PMID: 28267032.
- Cho YW, Kim E. Is stopping of anticoagulant therapy really required in a minor dental surgery? - How about in an endodontic microsurgery? Restor Dent Endod. 2013 Aug;38(3):113-8. Pubmed PMID: 24010076. ,/
- Englehart MS, Cho SD, Tieu BH, Morris MS, Underwood SJ, Karahan A, et al. A novel highly porous silica and chitosan-based hemostatic dressing is superior to HemCon and gauze sponges. J Trauma. 2008 Oct;65(4):884-90. Pubmed PMID: 18849807.
- Brennan MT, Valerin MA, Noll JL, Napeñas JJ, Kent ML, Fox PC, et al. Aspirin use and post-operative bleeding from dental extractions. J Dent Res. 2008;87(8):740-4. PubmedPMID: 18650545.
- Perry DJ, Noakes TJ, Helliwell PS; British Dental Society. Guidelines for the management of patients on oral anticoagulants requiring dental surgery. Br Dent J. 2007 Oct 13;203(7):389-93. Pubmed PMID: 17934422.