HSP47 as a Possible Molecular Chaperone for the Collagen Synthesis in the Mouse Periodontal Ligament Cells due to Orthodontic Force
Muraoka R1*, Nakano K2, Yamada K1, Kawakami T3
1 Department of Orthodontics, Matsumoto Dental University School of Dentistry, Shiojiri, Japan.
2 Department of Oral Pathology and Medicine, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama,
Japan.
3 Hard Tissue Pathology Unit, Matsumoto Dental University Graduate School of Oral Medicine, Shiojiri, Japan.
*Corresponding Author
Rina Muraoka,
Assistant Professor,
Department of Orthodontics,
Matsumoto Dental University School of Dentistry,
1780 Hirooka-Gobara, Shiojiri, 399-0781, Japan.
Tel: +81-263-51-2086
Fax: +81-263-51-2035
E-mail: mura@po.mdu.ac.jp
Received: December 30, 2016; Accepted: January 16, 2017; Published: January 17, 2017
Citation: Muraoka R, Nakano K, Yamada K, Kawakami T (2017) HSP47 as a Possible Molecular Chaperone for the Collagen Synthesis in the Mouse Periodontal Ligament Cells due to Orthodontic Force. Int J Dentistry Oral Sci. 4(1), 387-394. doi: dx.doi.org/10.19070/2377-8075-1700078
Copyright: Muraoka R© 2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Orthodontic mechanical stress induces various molecules, which cause structural changes in the proteins in periodontal tissue. The expression kinetics of HSP47 in mouse periodontal ligament (PDL) tissue after application of corrective mechanical stress and HSP47 expression during the PDL recovery were observed. Our examination data are as follows: HSP47 is constitutively expressed in the PDL and defends cells from different types of stress and maintains homeostasis under normal conditions. HSP47 is expressed in PDL fibroblasts on the pressure side damaged by application of mechanical stress and contributes to the repair of collagen tissue by activating PDL fibroblasts, supporting recovery from cell damage. These data suggests that, during bone addition to the PDL on the tension side, HSP47 also acts as molecular chaperone, which assists the maturation of bone morphogenetic proteins and aids osteoblast activation. On the other hand, it is possible that, when a mechanical stress is applied to the periodontal membrane on the tension side for a time too short for bone addition and abnormalities are caused in the collagen structure of the PDL fibroblasts, by functioning at the damage site, HSP47 inhibits extracellular secretion of abnormal collagen, stores the modified protein in the endoplasmic reticulum, thereby controlling decalcification of the PDL. HSP47 investigated in this study acts differently depending of the time of expression.
2.Introduction
3.Materials and Methods
3.1.Experimental Animals
3.2.Experimental Methods
3.3.Immunohistological Investigation of HSP47
4.Results
4.1.Control Group
4.2.Experimental Group
5.Discussion
6.Acknowledgment
7.References
Keywords
HSP47; Molecular Chaperone; Collagen; Periodontal Ligament Cells; Mechanical Stress; Orthodontic Tooth Movement.
Introduction
Orthodontic tooth movement requires alteration or restructuring of the periodontal tissue. In orthodontic treatment, remodeling of the periodontal tissue is brought about by mechanical stress due to a correction device and, because of tissue responses to this mechanical stress, the tooth moves [1]. The periodontal tissue responds to different types of stimulus such as mechanical stress and inflammation, to maintain homeostasis, and expresses various proteins to bring about active remodeling of the periodontal tissue [2-11]. It is important to grasp the kinetics of various factors expressed in the periodontal tissue during orthodontic treatment, as a biochemical basis. In recent years, many histological and cell differentiation studies and reports on various transcription factors, which regulate morphogenesis, have been reported [12-16].
Heat shock proteins (HSPs) are known as the principal proteins expressed in various organs and tissues in response to cytotoxic stimuli and mechanical stress [17, 18]. HSP are also induced by pathological changes such as inflammation, physical stress, and chemical stress as well as heat shock. HSPs are classified based on their molecular weight. However, the functions of each HSP remain unclear. HSPs are involved in the defense and repair of injured cells and are thought to contribute to the control of cellular function [19, 20]. However, while HSPs are involved in regulatory cellular responses to injurious stimuli, there are few reports regarding their expression state in periodontal ligament (PDL) tissue accompanying orthodontic tooth movement or their roles [2, 11, 16]. The principal constituent of the extracellular matrix of the PDL is collagen, which acts to alleviate mechanical stresses such as occlusal forces and mechanical corrective forces. The collagen fibers of the PDL are damaged by mechanical stress, and, once the mechanical stress is released, collagen fibers return to their original form. However, many points remain unclear with regards to the cellular proteins that contribute to the recovery mechanism. Accordingly, in this study, we focused on collagen synthesis and HSP47, which are essential to the development of mice and humans, and carried out an immunohistochemical investigation of HSP47 expression state in mouse PDL tissue after applying a mechanical stress and during the recovery of the PDL.
Seventy 8-week-old male ddY mice (body weight 35 ± 5 g) were sed in this study. The mice were kept in an environment controlled by an air conditioning unit, in metal cages with bedding on the floor (Paper clean: Peparlet Co., Ltd., Shizuoka, Japan).
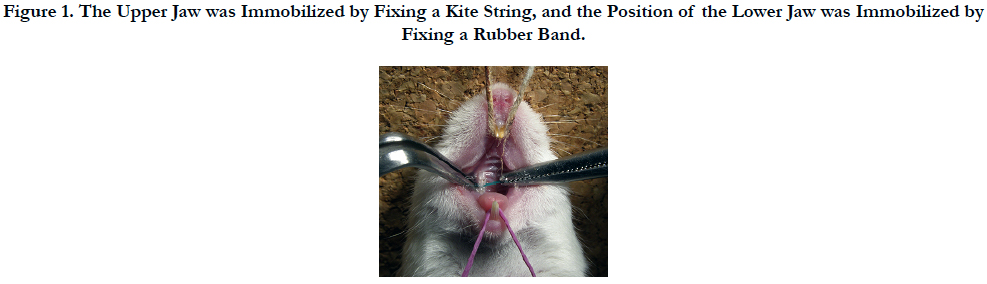
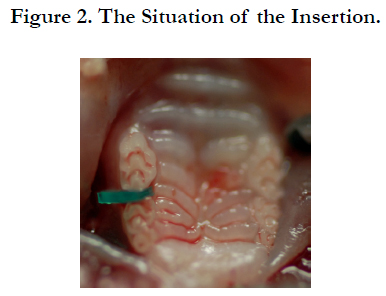
Before the experiments, anesthesia was induced in the mice by inhalation of a gaseous mixture of isoflurane (Isoflu: Dainippon Sumitomo Pharma Co., Ltd. Animal Science Division, Osaka, Japan) and air (pre-anesthetic concentration 4.0%). A small experimental animal gas anesthesia system, which enables constant regulation of gas anesthesia and flow speed (DS Pharma Biomedical Co., Ltd. Laboratory Products Division, Osaka, Japan), was employed in order to carry out the experiments under stably maintained anesthesia. After an anesthetic response, the upper body of the mice under general anesthesia was raised onto a handmade experimental platform and fixed in a seated position. General anesthesia was maintained during the experiments using an inhalation tube via the snouts of the mice (maintenance anesthetic concentration 1.0%). In order to keep the mouth of the mice opened during the experiments, the upper jaw was immobilized by fixing a kite string to the upper incisors of the mice from above the experimental platform, and the position of the lower jaw was immobilized by fixing a rubber band to the lower front teeth of the mice from below the experimental platform to pull the lower jaw downward. A separator was inserted by the method of Waldo [21] in order to apply a persistent mechanical stress to the upper molar PDL region of the mice (Figures 1 and 2). Rubber dam sheet (Heavy Force, Ivory, Premium Rubber Dam Pure Latex: Heraeus Kulzer GmbH & Co. KG, Hanau, Germany) cut in a square of 2.0 × 2.0 mm folded in two was used as a separator. The upper molar region of the mice is the sequence of three teeth, the first molar (M1), second molar (M2), and third molar (M3) from the mesial side. Accordingly, the separator was inserted between the upper right first molar (M1) and second molar (M2). In this study, HSP47 expression was evaluated by using two experimental systems.
Figure 1. The Upper Jaw was Immobilized by Fixing a Kite String, and the Position of the Lower Jaw was Immobilized by Fixing a Rubber Band.
First, a mechanical stress was applied over time by means of the separator. The mechanical stress was released after a time of up to 24 hours, and the upper molar periodontal tissue of the mice in the region in question was removed en bloc. There were 6 groups in this experiment, a group subjected for 10 minutes to mechanical stress (10 min), a 20-minute group (20 min), a 1-hour group (1h), a 3 hour group (3h), a 9-hour group (9h), and a 24-hour group (24h), with 5 animals per group (Table 1). It should be noted that, during the application of mechanical stress, the mice were only given water, in order to prevent the separator from falling out or coming loose.
After applying mechanical stress by means of a separator for a set period of 3 hours, the stress was released and the upper molar periodontal tissue of the mice was removed over time from immediately after the removal of the separator and from 20 minutes after removal to up to 1 week after removal. There were 8 groups in this experiment: a group in which the tissue was removed immediately after removing the separator (3 hours + 0 minute; 3 h + 0 min), a 20-minute after removing the separator group (3h + 20min), a 1-hour group (3h + 1h), a 3-hour group (3h + 3h), a 9-hour group (3 h + 9 h), a 24-hour group (3h + 24h), a 3-day group (3h + 3d), and a 1-week group (3h + 1w), with 5 animals per group (Table 2). It should be noted that, since the mechanical stress was applied for 3 hours and then released, the mice were allowed to consume both water and solid feed (Picolab Rodent Diet 20: Japan SLC, Inc., Hamamatsu, Japan) freely until the end of the experimental period.
The upper left molar periodontal tissue from the same individual mice (the opposite, untreated side) was used as a control group. For both the experimental and control groups, the distobuccal root of the upper first molar was the site observed in these experiments.
All animals used in this study were cared for in accordance with the experimental animal guidelines of the Matsumoto Dental University Animal Study Ethics Committee. The protocol for this study followed the Matsumoto Dental University animal study guidelines and was approved by Matsumoto Dental University Animal Study Ethics Committee (Approval No: 179-10).
In the two experiments described above, the murine upper molar periodontal tissue was excised together with the jawbone, promptly fixed, and immersed for 24 hours in a fixing solution (4% paraformaldehyde 0.05M phosphoric acid buffer). It was then demineralized for 3 weeks in a demineralizing solution (10% EDTA solution). Next, it was embedded in paraffin. A series of horizontal 5-μm thick sections was produced for the PDL region of the roots in question and immunohistochemically examined.
The immunohistological study was carried out using Dako Envision+ Kit K4006 (Dako, Glostrup, Denmark). An anti-rabbit HSP47 polyclonal antibody (HSP47 (H-300): sc8352, Santa Cruz Biotechnology, Inc., Dallas, TX, USA, 1/1000 dilution) was used as the primary antibody. The sample was counterstained with hematoxylin. The negative controls were treated by using the same experimental protocol, but without the primary antibody.
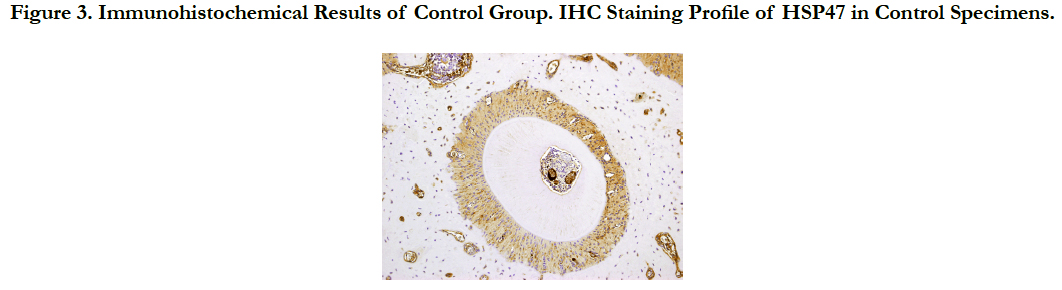
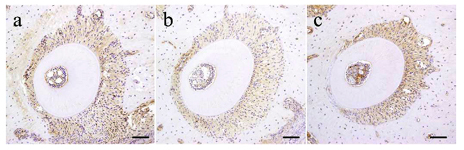
In the PDL tissue from rats in the control group (murine upper left first molar distobuccal root), HSP47 expression was detected in the cell cytoplasm of the PDL collagen bundles uniformly over the entire PDL (Figure. 3).
Figure 3. Immunohistochemical Results of Control Group. IHC Staining Profile of HSP47 in Control Specimens.
The distal side of the PDL on the side in which the separator was inserted (murine upper right first molar distobuccal root) was the tension side, and the proximal PDL on the opposite side was the pressure side.
The mouse upper molar periodontal tissue was removed after applying mechanical stress over time up to 24 hours by inserting a separator and histological analysis of the mouse periodontal tissue was performed (Figure 4).
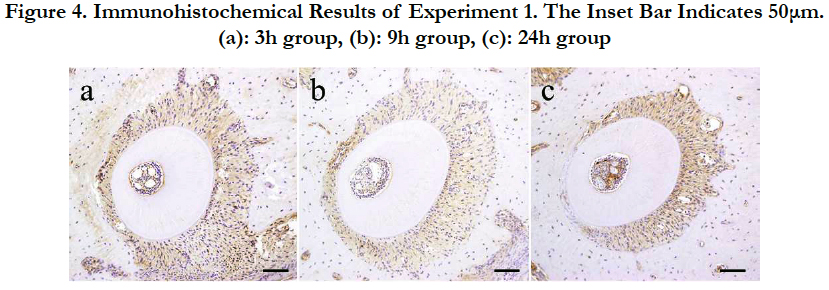
Figure 4. Immunohistochemical Results of Experiment 1. The Inset Bar Indicates 50μm. (a): 3h group, (b): 9h group, (c): 24h group.
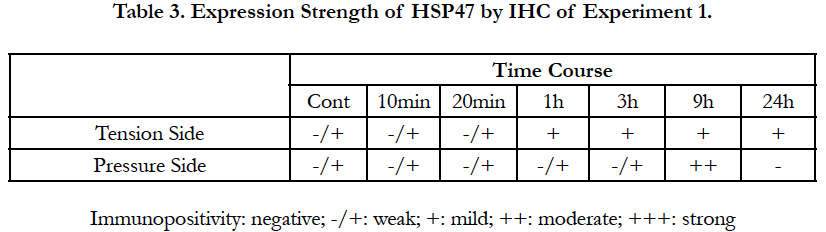
First, in the 10-min group, the position of the root in the alveolar socket was slightly moved proximally. However, the changes in the width of the PDL on the tension side and on the pressure side were not clear. There were hardly any differences in the intensity of HSP47 expression when compared with the control group.
In the 20-min group, the root moved proximally, and the widths of the PDL on the tension side and on the pressure side had become distinguishable. As in the 10-min group, the difference in the intensity of HSP47 expression was no clear difference.
In the 1-h group, the position of the root in the alveolar socket moved further proximally, and there was a clear difference between the width of the PDL on the tension side and the pressure side. In addition, HSP47 expression in the extended PDL on the tension side was more intense than that in the control group.
In the 3-h group, the relative proximal movement of the root advanced, the PDL space on the pressure side had shrunk and became narrow, the width of the PDL space on the tension side was further enlarged, and the PDL fibers in this region were markedly extended. In part of the PDL, the PDL fibers became sparse, and there were places in which the PDL fibers were broken, producing spaces. However, HSP47 expression on the tension side was more intense than that in the control group.
In the 9-h group, the enlarged width in the PDL on the tension side observed in the 3-h group was maintained. However, the intensity of HSP47 expression showed localized changes in the 9-h group. A more intense HSP47 expression was observed in the pressure side than that in the tension side.
In the 24-h group, an even more intense HSP47 response was observed in the PDL on the tension side. However, in the pressure side, HSP47 expression disappeared because the PDL fibroblasts were strongly compressed, but HSP47 expression was detected in the PDL fibroblasts adjoining the compressed PDL.
To summarize the results of Experiment 1, from a very early stage, in the 1-h group, a positive HSP47 response appeared in the PDL on the tension side, and the same level of expression was maintained for 9 hours. The intensity of HSP47 expression showed the greatest elevation in the 24-h group. The positive immune HSP47 response in the tension side showed a gradual increase in intensity over time. As for the positive immune HSP47 response in the pressure side, a low expression state was maintained, with no marked change in expression after applying stress, but a strong positive response was detected in the 9-h group. However, in the 24-h group, HSP47 expression disappeared in the pressure side, because the PDL fibroblasts were strongly compressed, but intense HSP47 expression was detected in the PDL fibroblasts adjoining the compressed PDL.
Next, mechanical stress was applied for 3 hours and then released, and the mouse upper molar periodontal tissue was removed over time for up to 1 week. Immunohistochemical analysis of HSP47 expression over time in mouse PDL tissue in the region in question was performed.
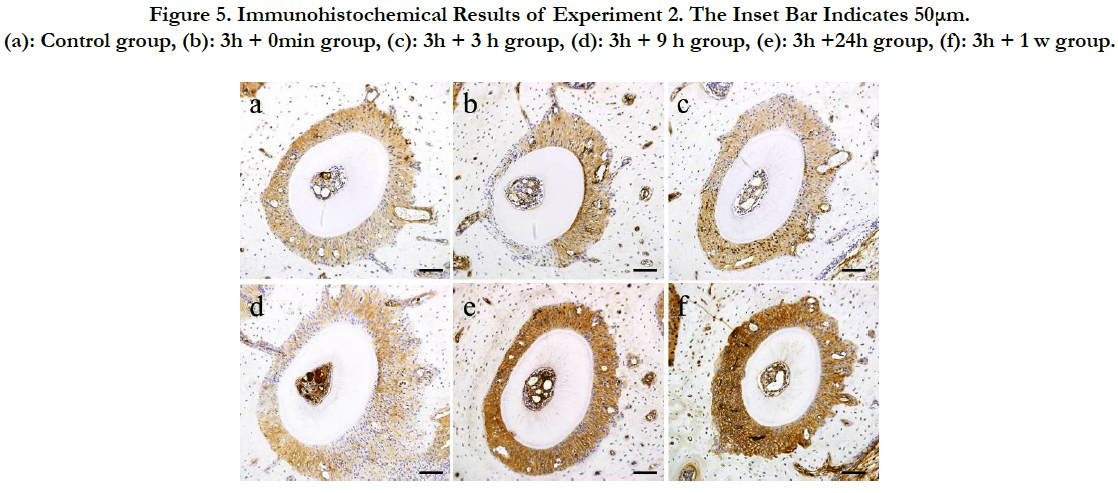
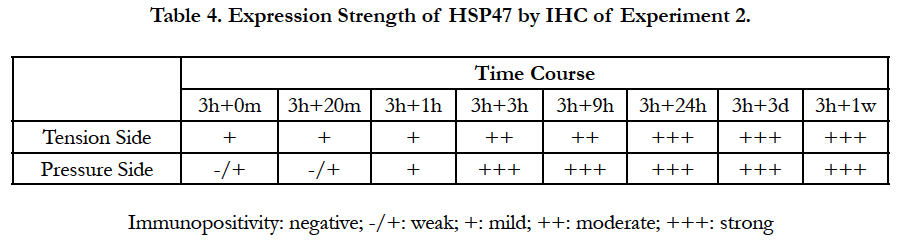
First, a mechanical stress was applied for 3 hours and then local changes in HSP47 expression were observed in the mouse PDL on the tension side and on the pressure side immediately after removing the separator in the 3 h + 0 min group. HSP47 expression in the PDL on the tension side was more intense than that in the control group. HSP47 expression in the pressure side was unchanged and remained weak.
In the 3h + 20 min group the intensity of HSP47 expression in the tension side and the pressure side was also similar to that in the 3h + 0 min group.
In the 3h + 1h group, the intensity of HSP47 expression in the PDL on the tension side was maintained compared to that in the 3h + 0 min and 3 h + 20 min groups. On the other hand, in the PDL on the pressure side, HSP47 expression increased, and there was no clear difference in the intensity of HSP47 expression between the pressure side and the tension side. HSP47 expression was similar in both sides.
In the 3h + 3h group, the position of the root in the alveolar socket had substantially returned to its initial position, and the width of the PDL was recovered. In addition, HSP47 expression in the pressure side and the tension side increased compared with that in the 3h + 1 h group. Moreover, HSP47 expression in the PDL on the pressure side was stronger than that in the PDL on the tension side.
In the 3h + 9h group, there was no change in the HSP47 expression. However, 24 hours after release (3h + 24h), HSP47 expression was again noticeably increased over the entire PDL. A positive HSP47 response was also noted in the osteoblasts and the bone cells of the alveolar bone. The same response was also observed 3 days after release (3h + 3d).
HSP47 expression remained strong over the entire PDL 1 week after release (3h + 1w). However, HSP47 expression was clearly weaker than that at 24 hours after release.
To summarize the results of Experiment 2, at a very early stage after releasing the mechanical stress, HSP47 expression increased in the PDL on the tension side compared to that in the control group, while HSP47 expression in the PDL on the pressure side was unchanged and remained weak. However, HSP47 expression gradually increased in the PDL on the pressure side and, 3 hour after release, HSP47 expression was stronger in the PDL on the pressure side than that in the PDL on the tension side. Nine hour after release, changes in HSP47 expression were suppressed. However, 24 hours after release, HSP47 expression was again noticeably increased over the entire PDL and HSP47 expression remained strong over the entire PDL until 1 week after release (Figure 5). These immunohistochemical results are summarized in Table 3 and Table 4.
Figure 5. Immunohistochemical Results of Experiment 2. The Inset Bar Indicates 50μm. (a): Control group, (b): 3h + 0min group, (c): 3h + 3 h group, (d): 3h + 9 h group, (e): 3h +24h group, (f): 3h + 1 w group.
Discussion
Fundamental research on the movement of teeth in orthodontic treatment in order to clarify aspects of bone absorption and addition and the underlying mechanism has a long history. In the past, we carried out animal experiments to establish an immunohistological basis for orthodontic treatment. We investigated various proteins expressed in the mouse periodontal tissue after application of mechanical stress simulating orthodontic treatment [2-11]. However, cellular responses related to repair of the injurious effects of mechanical stress on PDL fibroblasts during corrective treatment have barely been investigated. Accordingly, we focused on HSPs, which maintain cell homeostasis and are expressed to combat various types of cell damage.
HSPs are a class of proteins whose expression is intensified by stress [18]. HSPs are stress proteins induced by pathological changes such as inflammation, physical stress, and chemical stress as well as heat shock [10,11]. Stress responses are extremely well conserved from prokaryotes, which do not have an immune system, to higher animals such as humans, and function as a universal and basic defense mechanism, responsible for an initial biological defense response. HSPs are proteins widely distributed in cells in the equilibrium state even in the absence of stress, and earlier in vitro and in vivo experiments clearly showed that they are proteins essential for various cellular functions such as cell differentiation, proliferation, survival, and functional maintenance [19, 22, 23]. HSPs are polypeptides of ten to several hundred kDa, classified by molecular weight, and each has different functions. Many HSPs suppress protein modifications as well as repair of modified proteins. They are molecular chaperones [22] having a so-called anti-apoptosis function to escape cell death [19, 20].
A molecular chaperone is an auxiliary protein needed in order to produce a protein. The linguistic origin of the word chaperone is French. The basic meaning of the word chaperone is an older senior female household servant who accompanies a young unmarried woman on her social debut to give guidance on social etiquette. These molecular chaperones mediate correct folding of polypeptides and assembly into an oligomer structure by temporarily binding to proteins in the immature state and act to ensure that these processes proceed correctly. Chaperones assist the maturation into proteins provided with different functions. However, the detailed behavior of different types of HSPs, as molecular chaperones, in periodontal tissue is unclear.
The PDL is the fibrous connective tissue surrounding the dental root. It immobilizes the tooth in the jawbone and is regularly subjected to mechanical stresses such as occlusal pressure. The biological metabolic half-life of the PDL is extremely short, at 1 day. Many cell types constitute the PDL such as fibroblasts, osteoblasts, osteoclasts, cementoblasts, cementoclasts, epithelial cell rests of Malassez, mast cells, and macrophages. There are also blood vessels and nerves, and extracellular matrix proteins of collagen fibers, oxytalan fibers, and glycoproteins. With regards to the constituents of the periodontal membrane, type I collagen is the main constituent (90%), there is less type III collagen (10%), and type V collagen is present in very small quantities. Collagen is one of the most prevalent fibrous proteins in the body of animals. In vivo, collagen is formed by assembling multiple molecules. The collagen molecules, which constitute the units of collagen, comprise polypeptides of 1000 bound amino acids. Three α chains form a helix to form the collagen fibers.
We focused on the collagen fibers, which are major constituents of the periodontal membrane, and decided to investigate the expression kinetics of HSP47, which is a molecular chaperone specific for collagen, and is the only HSP localized in the endoplasmic reticulum in the PDL fibroblasts.
HSP47 defends cells from stress and supports intracellular collagen maturation and secretion. In addition, when normal synthesis fails because of different types of stress and abnormalities are caused in the collagen structure, HSP47 inhibits extracellular secretion of abnormal collagen, storing the modified protein in the endoplasmic reticulum. In 1986, Nagata et al., reported that the molecular chaperone essential for forming collagen with the correct 3 strands is HSP47 [24, 25]. In knockout mice lacking the collagen-specific molecular chaperone HSP47, abnormalities occur in the formation of collagen fibers comprising type I collagen. Abnormalities are observed in the basal membrane formation accompanying abnormalities in type IV collagen, and the mice are embryonic lethal [26, 27]. Thus, HSP47 is a molecular chaperone essential for normal development and tissue formation in mammals. With regards to HSP47 expression in the periodontal tissue, it has been reported that, in vitro, expression of HSP47, 60, and 70 intensifies when a periodic stretching force is appliedto human PDL fibroblasts [15]. Accordingly, it was thought that HSP47 would be similarly expressed in periodontal tissue in vivo during experimental orthodontic tooth movement.
Expression of HSP peptides occurs within an extremely short time in PDL cells subjected to mechanical stress over time [2-6, 8-10]. Accordingly, HSP47, which contributes to cell differentiation, is also expressed in a comparatively short time and changes in the intensity of HSP47 expression could be observed within 24 hours. The experimental periods of these experiments were set to enable comparison with the data obtained by Watanabe et al., [2-4] Matsuda et al., [6, 10], and prior reports [5, 8, 9, 11], from 10 minutes to a maximum time of 24 hours after inserting a rubber dam sheet. Moreover, in order to investigate the recovery of the periodontal tissue from damage due to mechanical stress, after applying a mechanical stress to the mouse PDL tissue for only 3 hours, HSP47 expression was observed over time up to a maximum time of 1 week after releasing the stress.
Our results show that, in the ligament tissue in the control group (distal buccal root of untreated mouse upper left first molar), HSP47 expression was noted in the cell cytoplasm of the PDL collagen bundles uniformly over the entire periodontal membrane and remained low. Under normal circumstances, teeth are subjected to mechanical stress due to mastication several thousand times daily. Under these circumstances, the supporting PDL tissue maintains its physiological functions. These findings in the control group PDL agree with reports that other HSPs such as HSP27 and HSP70 are present even in the absence of stress [19, 28, 29] and act to maintain physiological functions in the PDL tissue [5, 8, 9]. It appears that, like these proteins, HSP47 is also expressed in the absence of stress and serves as an element of the mechanism underlying the physiological functions of the PDL tissue and maintains the homeostasis of the PDL.
Next, in the experimental groups subjected to mechanical stress over time for up to 24 hours, HSP47 expression was detected in the PDL on the tension side from a very early stage and gradually increased over time with the greatest increase in the 24 hour group. On the pressure side, no marked change was detected in HSP47 expression after applying a stress, and low expression was maintained. However, a strong positive HSP47 response was observed in the group to which stress was applied for 9 hours. However, in the pressure side in the 24 hour group, HSP47 expression was absent because the PDL fibroblasts were strongly compressed, but intense HSP47 expression was detected in PDL fibroblasts adjoining the compressed PDL. When examining the time course of HSP47 expression after applying a mechanical stress, HSP47 expression occurred with the same timing as the expression of proteins such as Runx2, Msx2, ALP, BMP, Smad, and P-Smad reported by Watanabe et al., and Matsuda et al., which contribute to controlling bone formation by activating osteoblasts [2, 4, 6, 10]. These data suggest that, during bone addition to the PDL on the tension side, HSP47 also has a molecular chaperone function, assisting the maturation of bone morphogenetic proteins and supporting osteoblast activation.
Next, we determined HSP47 expression at different time points after applying a mechanical stress for only 3 hours to mouse the PDL tissue and releasing the stress up to a maximum time of 1 week. At a very early stage after releasing the mechanical stress, HSP47 was highly expressed in the PDL on the tension side, while its expression in the periodontal membrane on the pressure side remained weak. However, as time passed, the width of the compressed PDL returned to its initial width before applying stress, and HSP47 expression in the periodontal membrane on the pressure side increased; and 3 hours after release, when the position of the root in the alveolar bone had almost returned to the initial state, HSP47 expression was stronger in the periodontal membrane on the pressure side than that in the periodontal membrane on the tension side. Twenty-four hours after release, HSP47 expression notably increased over the entire PDL, and HSP47 expression remained at a high level over the entire PDL until 1 week after release (3h + 1w). These responses are thought to have been induced by the mechanical stress on the PDL. We previously reported that HSP70, expressed in a pressure side, which has been subjected to intense cell damage, may contribute to osteoclast differentiation on the pressure side, suppresses modification of nascent protein therein, and fulfills the function of carrying out management and repair of modified proteins, which cannot be regenerated [9]. It is conjectured that HSP47, which, like HSP70, has a function in tissue repair, is expressed in PDL fibroblasts on the pressure side damaged by applying a mechanical stress, contributes to repairing collagen tissue by activating PDL fibroblasts, and contributes to the recovery from cell damage. Moreover, as previously mentioned, HSP47, expressed in PDL fibroblasts on the tension side probably has a molecular chaperone function, which assists the maturation of bone morphogenetic proteins and aids osteoblast activation. However, it is possible that, when a mechanical stress is applied to the periodontal membrane on the tension side for a time that is not sufficient for bone addition, cell damage is produced and abnormalities are caused in the collagen structure of the PDL fibroblasts. However, by functioning at the damage site, HSP47 inhibits extracellular secretion of abnormal collagen and stores the modified protein in the endoplasmic reticulum, thereby controlling the decalcification, which is characteristic of PDL.
In summary, orthodontic mechanical stress induces various molecules, which cause structural changes in the proteins in periodontal tissue. The expression kinetics of HSP47 in mouse periodontal ligament (PDL) tissue after application of corrective mechanical stress and HSP47 expression during the PDL recovery were observed. Regarding our examination, an orthodontic mechanical stress was applied to mouse PDL tissue, and the expression kinetics of HSP47 over time in the PDL tissue subjected to stress continuously for up to 24 hours, and HSP47 expression during the PDL recovery after applying a mechanical stress to mouse PDL tissue for only 3 hours and then releasing it, were thoroughly investigated from an immunohistochemical point of view. We obtained the following results. In PDL fibroblasts from the control group, uniform low HSP47 expression was noted over the entire PDL. In PDL tissue from the experimental groups, with a mechanical stress load applied for up to 24 hours, a positive HSP47 response appeared in the PDL on the tension side from a very early stage, after applying stress for 1 hour, and the same level of expression was subsequently maintained for up to 9 hours of stress application. The expression was the highest in the 24-hour group. This positive immune HSP47 response on the tension side showed a gradual increase in intensity over time. In the pressure side, a marked change in expression was not observed after applying stress, and a low expression state was maintained, but a strong positive response was observed in the group with a stress load applied for 9 hours. However, in the 24-hour group, HSP47 expression disappeared in the pressure side because the PDL fibroblasts were strongly compressed, but intense HSP47 expression was detected in PDL fibroblasts adjoining the compressed PDL. Next, when a mechanical stress was applied for 3 hours, the periodontal tissue of the mouse upper molar region was allowed to recover for a maximum of 1 week after releasing the load. A very short time after releasing the mechanical stress, HSP47 expression in the PDL on the tension side was more intense than that in the control group. HSP47 expression in the PDL on the pressure side was unchanged and remained low. However, in the 1-hour after elease group, HSP47 expression increased in the PDL on the pressure side, and in the 3-hour after release group, the intensity of HSP47 expression in the PDL on the pressure side was more intense than that in the PDL on the tension side. In the 9-hour after release group, increases and decreases in HSP47 expression were temporarily suppressed, but, 24 hours after release, HSP47 expression was again noticeably increased over the entire PDL. HSP47 expression remained strong over the entire PDL until 1 week after release. Thus, HSP47 is constitutively expressed even in the absence of stress and is thought to contribute to the maintenance of homeostasis by defending cells from different types of stress. In mouse PDL fibroblasts, HSP47 expression is increased by applying a mechanical stress, which entails intense damage to the PDL fibroblasts, and contributes to collagen tissue repair by activating the PDL fibroblasts, supporting recovery from cell damage. It has been suggested that, when bone addition takes place in the PDL on the tension side, HSP47 also has a molecular chaperone function, which assists the maturation of bone morphogenetic proteins and aids osteoblast activation. On the other hand, when damage is induced to the collagen structure of PDL fibroblasts by applying a mechanical stress to the PDL on the tension side for a period of only 1 hour, without leading to bone addition, it is possible that HSP47 controls the decalcification, a PDL characteristic, by functioning at the site of the damage to inhibit extracellular secretion of abnormal collagen, supporting storage of the modified protein in the endoplasmic reticulum. Our data suggest that HSP47 acts differently depending on the time of expression.
Acknowledgement
This research was partially supported in part by JSPS KAKENHI Grant Numbers 26861804, 26463104, 25463204 from the Japan Society for the Promotion of Science (JSPS).
References
- Krishnan V, Davidovitch Z (2006) Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop1. 129(49): 469.e1-32.
- Watanabe T, Nakano K, Muraoka R, Shimizu T, Okafuji N, et al., (2007) Role of Msx2 as a promoting factor for Runx2 at the periodontal tension sides elicited by mechanical stress. Eur J Med Res. 13(9): 425-431.
- Watanabe T, Okafuji N, Nakano K, Shimizu T, Muraoka R, et al., (2007) Periodontal tissue reaction to mechanical stress in mice. J Hard Tissue Biol. 16(2): 71-74.
- Watanabe T, Nakano K, Shimizu T, Okafuji N, Kurihara S, et al., (2009) Immunohistochemistry of the periodontal ligament fibroblasts in orthodontic tension sides. J Hard Tissue Biol. 18(4): 175-180.
- Muraoka R, Nakano K, Matsuda H, Tomoda M, Okafuji N, et al., (2009) Immunohistochemical observation of heat shock proteins expression in mouse periodontal tissues due to orthodontic mechanical stress. J Hard Tissue Biol. 18(4): 193-197.
- Matsuda H, Muraoka R, Tomoda M, Nakano K, Okafuji N, et al., (2009) Immunohistochemical observation of BMP in the mouse orthodontic periodontal tension sides. J Hard Tissue Biol. 18(4): 181-184.
- Kawakami T, Nakano K, Shimizu T, Kimura A, Okafuji N, et al., (2009) Histopathological and immunohistochemical background of orthodontic treatment. Int J Med Biol Front. 15(7/8): 591-615.
- Muraoka R, Nakano K, Kurihara S, Yamada K, Kawakami T (2010) Immunohistochemical expression of heat shock proteins in the mouse periodontal tissues due to orthodontic mechanical stress. Eur J Med Res. 15(11): 475-482.
- Muraoka R, Nakano K, Matsuda H, Tomoda M, Okafuji N, et al., (2011) A consideration on the role of HSP70 appearing in the periodontal tissues due to experimental orthodontic force. J Hard Tissue Biol. 20(4): 275-282.
- Matsuda H, Harada T, Muraoka R, Tomoda M, Okafuji N (2011) Immunohistochemical observation of Osterix appearing in the mouse orthodontic periodontal tissues. J Hard Tissue Biol. 20(4): 283-288.
- Tomoda M, Nakano K, Muraoka R, Matsuda H, Yamada K, et al., (2012) Immunohistochemical changes of heat shock protein 27 expression in the mouse periodontal tissues exposed to orthodontic mechanical stress. J Hard Tissue Biol. 21(1): 43-50.
- Muraoka R, Tsujigiwa H, Nakano K, Katase N, Tamamura R, et al., (2011) Transplanted bone marrow-derived cell migration into periodontal tissues and cell differentiation. J Hard Tissue Biol. 20(4): 301-306.
- Tomida M, Tsujigiwa H, Nakano K, Muraoka R, Nakamura T, et al., (2013) Promotion of transplanted bone marrow-derived cell migration into the periodontal tissues due to orthodontic mechanical stress. Int J Med Sci. 10(10): 1321-1326.
- Maeda T, Kameda T, Kameda A (1997) Loading of continuously applied compressive force enhances production of heat shock protein 60, 70 and 90 in human periodontal ligament-derived fibroblast-like cells. J Jpn Orthod-Soc. 56: 296-302.
- Okazaki M, Shimizu Y, Chiba M, Mitani H (2000) Expression of heat shock proteins induced by cyclical stretching stress in human periodontal ligament fibroblasts. Tohoku Univ Dent J. 19(2): 108-115.
- Arai C, Nomura Y, Ishikawa M, Noda K, Choi JW, et al., (2010) HSPA1A is upregulated in periodontal ligament at early stage of tooth movement in rats. Histochem Cell Biol. 134(4): 337-343.
- Ritossa F (1962) A new puffing pattern induced by temperature shock and DNP in drosophila. Cell Molecul. 18(12): 571-573.
- Milton JS (1990) Heat shock proteins. J BiolChem. 265(21): 12111-12114.
- Lindquist S, Craig EA (1988) The heat-shock proteins. Annu Rev Genet. 22: 631-677.
- Arrigo AP, Landry J (1994) Expression and function of the low molecular weight heat shock proteins. The Biology of Heat Shock Proteins and Molecular Chaperones. Cold Spring Harbor Laboratory Press, North America, 335-373.
- Waldo CM (1953) Method for the study of tissue response to tooth movement. J Dent Res. 32: 690-691.
- Hratl FU (1996) Molecular chaperone in cellular protein folding. Nature. 381(6583): 571-579.
- Sakurai Y, Okuyama N, Tamamura K, Owawa R, Ito H , et al., (2007) Expression of collagen-specific stress protein Hsp47 in rat epithelial tissue. Ohu Univ Dent J. 34(4): 131-136.
- Nagata K, Saga S, Yamada KM (1986) A major collagen-binding protein of chick embryo fibroblasts is a novel heat shock protein. J Cell Biol. 103(1): 223-229.
- Nagata K, Saga S, Yamada KM (1988) Characterization of a novel transformation- sensitive heat-shock protein (HSP47) that binds to collagen. Biochem Biophys Res Comm. 153(1): 428-434.
- Nagai N, Yorihuzi T, Hosokawa N, Nagata K (1999) Human genome has a functional hsp47 gene (CBP2) and pseudogene (pshsp47). Gene. 227(2): 241-248.
- Marutani T, Yamamoto A, Nagai N, Kubota H, Nagata K (2004) Accumulation of type IV collagen in dilated ER leads to apoptosis in Hsp47-knockout mouse embryos via induction of CHOP. J Cell Sci. 117(pt 24): 5913-5922.
- Tsujimura K, Morishita M, Kawahara K, Fukunaga M, Tsuruda K, et al., (1995) A study on the expression of heat shock protein genes in the human periodontal ligament fibroblasts. J Jpn Soc Periodontol. 37(2): 287-293.
- Yamashita S, Maeshima A, Nojima Y (2005) Involvement of renal progenitor tubular cells in epithelial-mesenchymal transition in fibrotic rat kidneys. J Am Soc Nephrol. 16(7): 2044-2051.