A Case Report of Systemic Sclerosis Complicated by Biventricular Heart Failure, Pulmonary Hypertension and Review of Literature
Akinboro AO1*, Akintunde AA2, Akinlade M2, Audu MB2
1 Dermatology Unit, Department of Internal Medicine, Ladoke Akintola University of Technology, Ogbomoso and LAUTECH Teaching Hospital, Ogbomoso, Oyo State, Nigeria.
2 Cardiology Unit, Department of Internal Medicine, Ladoke Akintola University of Technology, Ogbomoso and LAUTECH Teaching Hospital, Ogbomoso, Oyo State, Nigeria.
*Corresponding Author
Dr. Adeolu Oladayo Akinboro
Dermatology Unit, Department of Internal Medicine,
Ladoke Akintola University of Technology,
Ogbomoso and LAUTECH Teaching Hospital, Ogbomoso, Oyo State, Nigeria.
Tel: + 234 813 6872 240
E-mail: deolusteve111@yahoo.com
Received: August 14, 2017; Accepted: October 07, 2017; Published: October 10, 2017
Citation: Akinboro AO, Akintunde AA, Akinlade M, Audu MB. A Case Report of Systemic Sclerosis Complicated by Biventricular Heart Failure, Pulmonary Hypertension and Review of Literature. Int J Cardiol Res. 2017;4(3):91-94. doi: http://dx.doi.org/10.19070/2470-4563-1700015
Copyright: Akinboro AO© 2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Systemic sclerosis (SSc) is an autoimmune connective tissue disorder whose aetiology is not fully understood. Skin fibrosis and visceral organs involvement are the hallmarks, and the heart could be disproportionately or subtly involved. Cardiovascular manifestations could include arrhythmias, pericarditis, myocardial fibrosis, pulmonary hypertension and congestive heart failure. Pulmonary involvement could cause lung fibrosis, arteriolar thickening, and pulmonary hypertension. The presence of these manifestations confers a poor prognosis.
Case Report: The case report presents a 50-year-old male patient with generalized skin sclerosis, hypo and hyperpigmented skin lesions, dyspnoea at rest, displaced apical impulse, tachycardia and pan-systolic murmur at the apex radiating to the axilla. Echo cardiography features showed ejection fraction of 33.3% with dilated atrial and ventricular chambers.
Conclusions: We present a case systemic sclerosis complicated by biventricular heart failure and pulmonary hypertension.
2.Introduction
3.Case Report
4. Discussion
5. Conclusions
6.References
KeyWords
Systemic Sclerosis; Heart Failure; Pulmonary Hypertension.
Introduction
Systemic sclerosis (SSc) is a relatively rare connective tissue disorder of unknown aetiology, with an incidence ranging from 2 to 20 per million population per year and a female-to-male ratio of roughly 3:1 [1, 2]. Systemic sclerosis is characterised by the skin and visceral organs fibrosis, in which the heart is frequently and severely involved. Studies have shown that up to 40 to 70% of patients with SSc may be harbouring one form of cardiac complication or the others [1, 3]. Sclerodermic heart disease, alone or in association with interstitial lung fibrosis and pulmonary hypertension, is one of the main determinants of overall prognosis in SSc [4].
Primary cardiac involvement develops as a direct consequence of SSc involvement of the heart in myocardial fibrosis may manifest as myocardial damage, fibrosis of the conduction system, pericardial and, less frequently, as valvular disease [5]. Also, cardiac complications in SSc may develop as a secondary phenomenon due to pulmonary arterial hypertension and renal crises [5].
Diffused sclerodermatous alteration describes the classic picture of SSc patient with visceral organ involvement or limited cutaneous form. Here we present the case of a 50-yearold Nigerian with advanced systemic sclerosis complicated by pulmonary hypertension and biventricular heart failure.
Case Report
50 years old roadside automobile mechanic who had a history of consistent contact with automobile oil and vibrating equipment presented to our clinic. His presenting symptoms include initial generalised body swelling and progressive hardening and dyspigmentation of the scalp, face, chest, the back, and the upper and lower limbs in the preceding two years. A year following, the patient presented to the clinic with progressively worsening dyspnoea, poor effort tolerance, later orthopnoea, paroxysmal nocturnal dyspnoea and bilateral leg swelling up to the knee.
There were no symptoms suggestive of renal failure. He was not on any cardiotoxic medication. The patient had no previous history of systemic hypertension, diabetes melitus and no family history of heart disease.
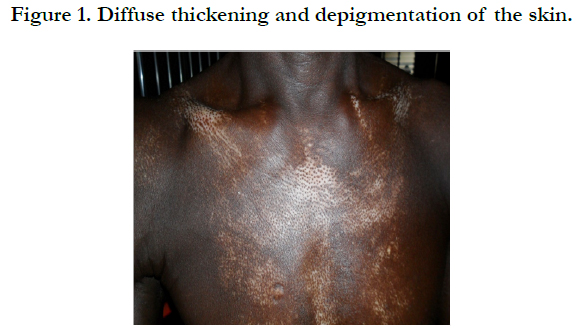
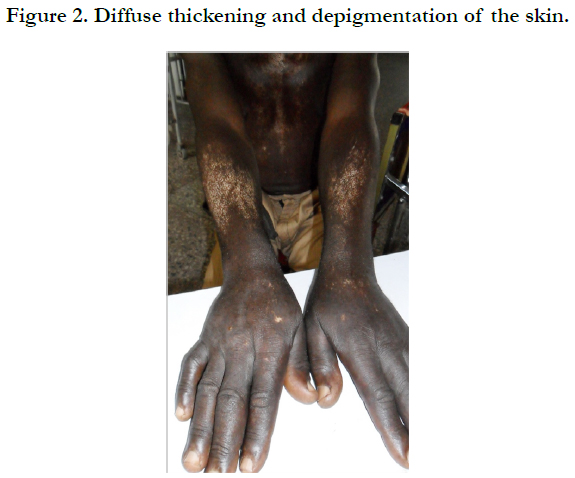
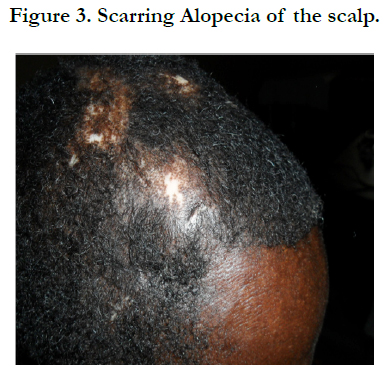
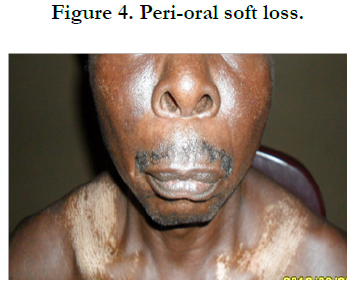
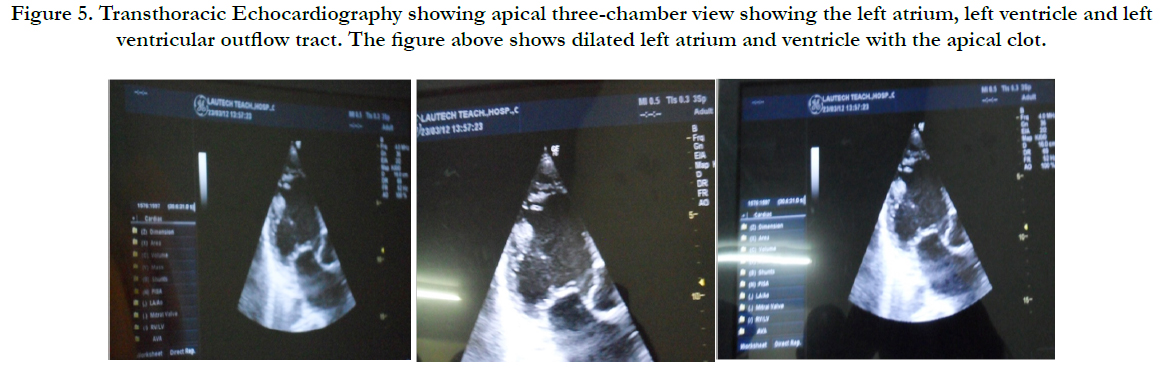
Physical examination revealed bilateral pitting pedal oedema up to the knee, skin dyspigmentation (hypo and hyperpigmented lesions) on the scalp with scarring alopecia, face, and chest, back, extensor surfaces of both forearms and legs (Figures 1, 2 and 3). There were sclerodactyly and loss of pulp substance of the digits. Patchy scarring alopecia of the frontal and vertex region was noticed. Patient’s mouth was deformed with peri-oral loss of soft tissue (Figure 4). Cardiac examination revealed tachycardia; blood pressure was 90/60mmHg; the maximum impulse was displaced laterally on sixth intercostal space anterior axillary line. Heart sound was normal with a pan systolic murmur. The anti-nuclear antibody testing was positive-homo and speckled pattern, and Scl-70 antibody was positive. Chest x-ray revealed cardiomegaly without evidence of lung fibrosis. The hilar vessels were engorged with the upward diversion of pulmonary vessels. There was blunting of costophrenic recesses. Echocardiography revealed; LVDD:55.9mm, LVSD:46.9mm, RVD:36.6mm, LAD:45.5mm, IVST:9.7mm, PWTd: 9.0mm, AOD:31.7mm, and ACS:20.0mm. The left atrium and ventricles were dilated with reduced biventricular systolic function, global hypokinesia, left ventricular apical clots, and generalised spontaneous echo contrast. The right ventricular outflow tract was dilated, severe tricuspid, pulmonary and mitral regurgitation was noted. Left ventricular ejection fraction was 33.35%, pulmonary arterial systolic pressure 33+10mmHg, dilated hepatic veins with reduced respiratory excursion compatible with pulmonary hypertension with biventricular failure and left ventricular intracardiac apical clot (Figure 5). Electrocardiography showed low limb leads voltage, LAE, PR interval-0.20 seconds, displaced transition zone (left), and ST-segment elevation in anterolateral leads.
Figure 5. Apical three-chamber view Transthoracic Echocardiography showing the left atrium, left ventricle, left ventricular outflow tract, dilated left atrium and ventricle with the apical clot.
Hematology reports showed Haemoglobin of 10.3g/dl, ESR-32mmol/hr (Westergren method). Electrolyte, urea, and creatinine were within normal limits. An assessment of biventricular heart failure in a patient with SSc-associated PAH was made, with NYHA class III dyspnoea. Treatment for congestive cardiac failure was optimised with furosemide, lisinopril, spironolactone, digoxin, and warfarin, with the aim of achieving an international normalised ratio of between 2-3. The patient was also placed on tab hydroxychloroquine. He was given a referral to the Rheumatologist and Cardiologist. The patient was doing well on these medications until he became non-compliant. He eventually had a cardioembolic stroke and died about two years after discontinuation of care with the specialists.
Discussion
Cardiac involvement in SSc can be categorized as involving the: myocardium, conduction system, pericardium, blood vessels [6] and the indirect effect following the development of pulmonary hypertension and renal crisis [7]. Both cardiovascular and pulmonary complications are responsible for the bulk of morbidities and mortalities associated with SSc [5-7].
The clinical presentation varies extensively in patients with SSc and cardiac involvement [5-7]. Our patient presents with a combination of the symptoms of right and left heart failure. These include pulmonary congestion, dyspnoea with exertion, paroxysms of nocturnal dyspnoea, orthopnoea, and pedal oedema. The indolent nature of dyspnoea and poor effort tolerance might be unconnected with the initial development of pulmonary hypertension and subsequent right heart failure and congestion. Orthopnea or paroxysms of nocturnal dyspnoea are unusual symptoms in the absence of left heart involvement. The other features of severe pulmonary hypertension not manifested by this patient are chest pain, dizziness, palpitations,syncope and sudden cardiac death following the development of arrhythmias or acute right ventricular failure [2, 8, 9].
Other findings that suggest the involvement of other components of the heart in this patient is the presence of an asymptomatic minimal pericardial effusion. Pericarditis could present with pericardial effusion or uremic pericarditis if there is associated scleroderma renal disease. Acute pericarditis in SSc has also been documented to present with diffuse ST-segment elevation [10]. Patients with arrhythmias may experience palpitations due either to bradycardia or tachycardia [4].
In the index case, doppler echocardiography shows evidence of biventricular systolic dysfunction. Previous studies have shown both diastolic and systolic dysfunction could occur in SSc [1, 5-7, 11]. However, diastolic dysfunction is a common cardiac abnormality in patients with SSc compared to systolic dysfunction [5-7, 11]. Diastolic dysfunction is a non-invasive measure of myocardial fibrosis, and ventricular filling stiffness [6]. In a multicenter series of patients with SSc in France, the prevalence of systolic dysfunction was 1.4% while diastolic dysfunction was commoner at 17.7% as reported by Groote et al., [12]. Champion et al., reported systolic dysfunction incidence of 11-15% [7]. When present, systolic dysfunction has been related to the simultaneous presence of coronary artery disease or hypertensive heart disease [13]. Although our patient had electrocardiography evidence of anterolateral ischemia, he has no previous history of systemic hypertension, and coronary angiography was not done partly because of limitation of funds and its scarce availability in our environment. Our patient had digital ulcerations and reduction of pulp substance, an independent risk factor for depressed left ventricular ejection factor. Other independent risk factors associated with reduced left ventricular ejection (LVEF) include age, male gender, myositis, and lack of treatment with calcium blockers [14]. In difference to Allanore et al. study, the presence of reduced LVEF was not associated with typical cardiovascular risk factors but an indicator SSc severity and markers of microvasculopathy [13].
Another important observation in this report is the elevated pulmonary arterial pressure demonstrated with Doppler echocardiography without chest x-ray evidence of lung fibrosis. However, high-resolution chest computerized tomography and pulmonary function test could be not performed to accurately assess for lung fibrosis. The reported prevalence of PH in SSc varied widely. Condliffe et al., [15], 2009 reported a prevalence of 10% to 12%, and prevalence ranged from 4.9% and 26.7% depending on the diagnostic tools employed [8]. PH is considered regarded as a harbinger of increased morbidity and mortality in SSc [2, 9, 16]. Pulmonary hypertension in SSc may be associated with pulmonary fibrosis or may develop due to vascular narrowing or occlusion in cases with or without minimal pulmonary fibrosis [9]. The pathophysiologic consequence of this anomaly is the right heart failure [2, 9, 16]. The risk factors for PH in clinical practice include factors such as: decreased diffusion capacity of lungs for carbon monoxide (DLCO), increased ratio of forced vital capacity to DLCO, the presence of anti-centromere antibodies, anti-nuclear antibody (ANA) pattern, and increased disease duration [16]. The positive anti topoisomerase has also been associated with rapid progressive skin thickening, pulmonary fibrosis and renal crisis [17].
There was widespread regurgitant murmurs: tricuspid, mitral, pulmonary and aortic in the present patient and the valve regurgitations especially mitral and tricuspid could be secondarily related to the ventricular dysfunction in this patient. Previously published studies have suggested scleroderma rarely involve the valves [5-7, 18], but any valve could be involved [18-20]. Meune et al., [18] reported the presence of small masses similar to Libman-Sacks vegetations, aortitis, aortic regurgitation (AR), and an increased frequency of mitral valve prolapse. Other study confirmed mitral valve as the most commonly affected valve [19]. Faccini et al., [20] also reported the significant greater prevalence of valvular abnormalities within five years in-patient with diffuse SSc.
Conclusion
Cardiopulmonary involvement portends grave consequences for patients with systemic sclerosis. Pulmonary hypertension and cardiovascular dysfunctions either primary or secondary frequently complicates SSc and are probably under-recognised due to a shortage of funds or non-availability of advance investigation modalities in our environment. Early screening of patients with SSc using echocardiography and lung function test will go long way to significantly contribute to early recognition and treatment of affected patients.
References
- Follansbee WP, Miller TR, Curtiss EI, Orie JE, Bernstein RL, Kiernan JM, et al. A controlled clinicopathologic study of myocardial fibrosis in systemic sclerosis (scleroderma). J Rheumatol. 1990 May;17(5):656–62. PubMed PMID: 2359076.
- Koh E, Lee P, Gladman DD, Abu-Shakra M. Pulmonary hypertension in systemic sclerosis: an analysis of 17 patients. Br J Rheumatol. 1996 Oct;35(10):989–93. PubMed PMID: 8883438.
- Ferri C, Emdin M, Giuggioli D, Carpeggiani C, Maielli M, Varga A, Michelassi C, et al. Autonomic dysfunction in systemic sclerosis: time and frequency domain 24-hour heart rate variability analysis. Br J Rheum. 1997 Jun;36(6):669–76. PubMed PMID: 9236677.
- Ferri C, Bernini L, Bongiorni MG, Levorato D, Viegi G, Bravi P, et al. Noninvasive evaluation of cardiac dysrhythmias, and their relationship with multisystemic symptoms, in progressive systemic sclerosis patients. Arthritis Rheum. 1985 Nov;28(11):1259. PubMed PMID: 4063000.
- Lambova s. Cardiac manifestations in systemic sclerosis. World J cardiol. 2014 sep 26;6(9):993-1005. PubMed Central PMCID: PMC4176808.
- Parks JL, TaylorMH, Laura PP, Silver RM. Systemic Sclerosis and the Heart. Rheum Dis Clin N Am. 2014 Feb;40(1):87-102.
- Champion HC. The heart in scleroderma. Rheum Dis Clin North Am. 2008 Feb;34(1):181–90. PubMed Central PMCID: PMC2361099.
- Proudman SM, Stevens WM, Sahhar J, Celermajer D. Pulmonary arterial hypertension in systemic sclerosis: the need for early detection and treatment. Intern Med J. 2007;37(7):485-494. PubMed PMID: 17547726.
- Lambova S, Müller-Ladner U. Pulmonary arterial hypertension in systemic sclerosis. Autoimmun Rev. 2010 Sep;9(11):761-770. PubMed PMID: 20601197.
- Imazio M, Bobbio M, Cecchi E, Demarie D, Demichelis B, Pomari F, et al. Colchicine in addition to conventional therapy for acute pericarditis: results of the Colchicine for acute PEricarditis (COPE) trial. Circulation. 2005 Sep;112(13):2012–6. PubMed PMID: 16186437.
- Armstrong GP, Whalley GA, Doughty RN, Gamble GD, Flett SM, Tan PL, et al. Left ventricular function in scleroderma. Br J Rheumatol. 1996 Oct;35(10):983–8. PubMed PMID: 8883437.
- de Groote P, Gressin V, Hachulla E, Carpentier P, Guillevin L, Kahan A, et al., Evaluation of cardiac abnormalities by Doppler echocardiography in a large nationwide multicentric cohort of patients with systemic sclerosis. Ann Rheum Dis. 2008 Jan;67(1):31-36. PubMed PMID: 17267515.
- Maione S, Cuomo G, Giunta A, de Horatio L, Montagna G, Manguso F, et al. Echocardiographic alterations in systemicsclerosis: a longitudinal study. Semin Arthritis Rheum. 2005 Apr;34(5):721-744.
- Allanore Y, Meune C, Vonk MC, Airo P, Hachulla E, Caramaschi P, et al. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis. 2010 Jan;69(1):218–21. PubMed PMID: 19279015.
- Condliffe R, Kiely DG, Peacock AJ, Corris PA, Gibbs JS, Vrapi F, et al. Connective tissue disease-associated pulmonary arterial hypertension in the modern treatment era. Am JRespirCrit Care Med. 2009 Jan 15;179(2):151–157. PubMed PMID: 18931333.
- Denton CP, Khanna D. Systemic Sclerosis. The lancet. 2017 Apr 13;Pii:S0140-6736(17)30933-9. PubMed PMID: 28413064.
- Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum. 2005 Aug;35(1):35-42. PubMed PMID: 16084222.
- Silveira-Torre LH. Cardiac involvement in systemic sclerosis. Reumatol Clin. 2006 Nov;2 Suppl 3:S31-6. PubMed PMID: 21794385.
- Meune C, Avouac J, Wahbi K, Cabanes L, Wipff J, Mouthon L, et al. Cardiac involvement in systemic sclerosis assessed by tissue-Doppler echocardiography during routine care: a controlled study of 100 consecutive patients. Arthritis Rheum. 2008 Jun;58(6):1803–1809. PubMed PMID: 18512815.
- Faccini A, Franchini S, Sabbadini MG, Camici PG. Cardiac involvement at rest in patients with systemic sclerosis: differences between the limited and the diffuse form of the disease. G Ital Cardiol (Rome). 2014 Jan;15(1):44-50.