The Prophylactic and Therapeutic Effects of Safranal and Selenite on Liver Damage Induced by Thyrotoxicosis in Adult Male Rats
Hussein RA1, El-Hussieny EA2, Hassanin LA1, El-Sayed WM2*
1 Hormonal Evaluation Department, National Organization for Drug Control & Research (NODCAR), Cairo, Egypt.
2 University of Ain Shams, Faculty of Science, Department of Zoology, Abbassia, Cairo, Egypt.
*Corresponding Author
Wael M. El-Sayed,
University of Ain Shams, Faculty of Science,
Department of Zoology, Abbassia, 11566, Cairo, Egypt.
Tel: +202/2482-1633
Fax: +202/2684-2123
E-mail: waelelhalawany@hotmail.com / wael_farag@sci.asu.edu.eg
Received: May 08, 2017; Accepted: May 24, 2017; Published: June 01, 2017
Citation: Hussein RM, El-Hussieny EA, Hassanin LA, El-Sayed WM (2017) The Prophylactic and Therapeutic Effects of Safranal and Selenite on Liver Damage Induced by Thyrotoxicosis in Adult Male Rats. Int J Clin Pharmacol Toxicol. 6(3), 270-279. doi: dx.doi.org/10.19070/2167-910X-1700045
Copyright: El-Sayed WM© 2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Aim: To evaluate the prophylactic and therapeutic activities of safranal and selenite against liver damage induced by thyrotoxicosis.
Materials and Methods: Thyrotoxicosis was induced by thyroxine (L-T4, 500 μg/kg, s.c.). Safranal (50 mg/kg, i.p.) or selenite (0.25 mg/kg, oral) was administered either with or after thyroxine. All treatments continued daily over three weeks.
Results: Treatment of rats with L-T4 resulted in significant elevations in fT3 and fT4 levels and significant reductions in the serum TSH level and body weight confirming the establishment of thyrotoxicosis. Thyrotoxicosis resulted in significant elevations in serum ALT and AST activities and hepatic levels of malondialdehyde and nitric oxide in addition to significant reductions in hepatic GSH level and the activities of catalase and SOD indicating the oxidative insult and depletion of antioxidants. Thyrotoxicosis also caused significant upregulations in the mRNA expression of bax, bax/bcl-2 ratio, and caspase-9 and a significant downregulation of bcl-2 in liver but did not affect the caspase-3 expression which would collectively induce apoptosis. Consequently, the DNA fragmentation was also increased and severe histopathological appearance of liver was reported. Safranal and selenite were able to mitigate all the previous injurious effects of thyrotoxicosis through bolstering the antioxidant defense systems.
Conclusions: The alleviation offered by safranal was better than that shown after selenite administration. Treating the hyperthyroid animals with safranal or selenite gave better palliating results than those reported for the protection regimen.
2.Introduction
3.Materials and Methods
3.1 Chemicals
3.2 Experimental Animals
3.3 Experimental Design
3.4 Blood Collection and Sample Analysis
3.5 Tissue and Sample Analysis
3.6 Biochemical Determinations
3.7 Assessment of bcl-2, bax, caspase-3 and caspase-9 expression by qRT-PCR
3.8 DNA Fragmentation % Assay
3.9 Histopathological Study
3.10 Statistical Analysis
4.Results and Discussion
5.Conclusion
6.References
Keywords
Thyrotoxicosis; Safranal; Selenite; Apoptosis; Antioxidants.
Introduction
Thyrotoxicosis is defined as the clinical manifestation of hyperthyroidism, a symptom with various adverse effects on the liver, heart, vascular, nervous and gastrointestinal systems. Thyrotoxicosis causes oxidative damage in various tissues such as liver, heart, and brain [1]. Liver damage in hyperthyroid patients is characterized by some degree of fatty infiltration, cytoplasmic vacuolization, nuclear irregularity, and hyperchromatism in hepatocytes but the pathogenesis of hepatic dysfunction in hyperthyroidism is uncertain. Possible thyroid–liver interactions include liver damage secondary to the systemic effects of excess thyroid hormones (THs), direct toxic effects of THs on the liver and changes in the metabolism of THs secondary to intrinsic liver disease [2]. Reactive oxygen species (ROS) play a role in the pathogenesis of tissue damage in many pathological conditions via the peroxidation of membrane phospholipids. Experimental studies suggest that hyperthyroidism is associated with an increase in free radical production and lipid peroxide levels [3].
Medicinal plants rich in antioxidant phytochemicals have received growing attention as potential preventive agents. These are recognized to exert their preventive effects by scavenging ROS and detoxifying potent genotoxic oxidants. Saffron is the dried stigmas of the flowers of Crocus sativus (family, Iridaceae). Safranal is one of the main active constituents of saffron. It has many pharmacological activities such as antioxidant, antitumor and anti-inflammatory effects. These activities have been attributed to its capability to stabilize bio-membranes, scavenge ROS, and decrease the peroxidation of unsaturated membrane lipids [4]. Selenium (Se) is an essential trace element that protects cell membranes from oxidative degradation and helps to overcome the oxidative stress by increasing the glutathione level leading to an increase in the cellular antioxidant milieu. Selenite was shown to be preventive against hepatotoxicity in rats [5].
The present work aimed at evaluating the prophylactic and therapeutic effects of safranal and selenite on the liver damage induced by thyrotoxicosis through assessing different oxidative/antioxidant parameters, apoptotic markers, and the tissue architecture.
Levothyroxine (L-T4 ) was purchased from Sigma (St. Louis, MO, USA). Safranal was purchased from Fluka Chemicals Ltd. (Old Brickyard, Gillingham, UK). Sodium selenite was obtained from Arab Company for Gelatin and Pharmaceutical Products (Amreya, Alexandria, Egypt). All other chemicals used were of analytical grade.
This study was carried out on adult male albino rats (Rattusnorvegicus) weighing 160-200g. Rats were obtained from the animal house of the National Organization for Drug Control and Research (NODCAR). The animals were kept under normal condition throughout the experiment. They were housed in a wire cage with natural ventilation and illumination and allowed free access to water and standard diet. Throughout the experiment, all the procedures and experimental protocols were approved by the Institutional Ethics Committee at NODCAR, and were carried out according to the criteria outlined in the “Guide for the care and use of the laboratory animals”.
After acclimatization, seventy two adult male albino rats were divided randomly into the following groups (8 rats/group); the first group received a daily subcutaneous (s.c.) injection of 0.5 ml isotonic alkaline saline (pH 8) for 3 weeks. The second group received a daily intraperitoneal (i.p.) injection of 0.25ml paraffin oil for 3 weeks. The third group received a daily i.p. dose of safranal dissolved in paraffin oil (50 mg/kg) for 3 weeks [4]. Group 4 received an oral daily administration of sodium selenite (0.25 mg/kg) for 3 weeks [5]. Group 5 received a daily s.c. dose of L-T4 dissolved in alkaline saline (500μg/kg) for 3 weeks [1, 2]. Group 6 was co-treated with L-T4 and safranal daily for 3 weeks. Group 7 was co-treated with L-T4 and sodium selenite daily for 3 weeks. Group 8 and 9 received L-T4 only for 2 weeks, then L-T4 and safranal or selenite, respectively for one week then animals were further treated with either safranal or selenite for another period of 2 weeks.
At the end of the experiment, animals were sacrificed by decapitation and the blood samples were collected in dry clean centrifuge tubes and allowed to clot for 30 min on ice then blood was centrifuged at 4,000 r.p.m for 10 min at 4ºC and serum was immediately separated and stored at -20 ºC till determination of thyroid hormones level and liver enzyme activities.
The liver was divided into three parts; part of the left lobe was immediately frozen in liquid nitrogen and kept at -80ºC until Reverse Transcription Polymerase Chain Reaction (RT- PCR) assay. The second part was taken from right lobe and kept in 10% formalin for histopathological examination. The remaining liver tissue was perfused using cold saline, excised and blot dried. The perfused liver was homogenized in ice-cold 1.15% KCl to make 10 % homogenate for biochemical determinations.
Serum levels of fT3 and fT4 were determined by competitive enzyme-linked immunosorbent assay (ELISA) using kits supplied from Biocheck, Inc. (Vintage Park Drive Foster city, USA). The thyroid stimulating hormone (TSH) was determined by sandwich enzyme-linked immunosorbent assay (ELISA) using a kit supplied from Biocheck, Inc. The color intensity was measured at 450 nm using a microplate reader (Biotech Power Wave Xs, USA). A standard curve was plotted for each parameter. Results were expressed as pg/ml for fT3, ng/dl for fT4 and μIU/ml for TSH.Activities of AST and ALT in serum were estimated using kits supplied from Randox laboratories limited (County Antrim, UK). Protein was determined according to the method of Lowry et al., [6]. MDA was measured as described before [7]. The level of MDA in the samples was expressed as nmol/100 g tissue using a standard curve. The concentration of NO was measured as described elsewhere [8]. NO concentration was expressed as μmol/g tissue. GSH content in liver homogenate was determined according to the method of VanDoorn et al., [9]. GSH was expressed as mmol/g tissue. SOD activity was estimated as described before [10]. The rate of increase in the absorbance at 420 nm was recorded for 3 min. The percent of inhibition was calculated as follows:
% inhibition =100 × [(ΔA/min blank - ΔA/ min sample) / (ΔA/ min blank)].
The one unit of SOD activity is defined as the amount of enzyme that inhibits the rate of pyrogallol auto-oxidation by 50%. The specific enzymatic activity was expressed as U/100 mg protein. The catalase assay was performed according to the method of Aebi [11]. Catalase activity was expressed as U/mg protein.
Total RNA was isolated from liver samples using BIOZOL reagent purchased from Bioer Technology Co., Ltd. (Hangzhou, China) following manufacturer’s instructions. Reverse transcription (RT) of total RNA to cDNA was performed by mixing 1μg of total RNA, 1.5 μl from 10μΜ oligo (dT) (Thermo Fisher Scientific Inc., Massachusetts, USA), 2 μl of 10 mMd NTP mix (Promega Corporation, Wisconsin, USA), 4 μl of 25mM MgCl2 (Promega Corporation), 2 μl of 10x RT buffer (SibEnzyme Ltd, Novosibirsk, Russia), 2μl RNase-inhibitor (Promega Corporation), 7.5 μl RT-enzyme (M-Mul V) (SibEnzyme Ltd.). The volume of this reaction mixture was completed to 25 μl of DEPCtreated water and incubated at 70°C for 10 min, then at 37°C for 10min, and 42°C for 1 hour, followed by final extension stage at 72˚C for 10 min. RT was carried out in Biometra thermocycler (Analytik Jena Company, Göttingen, Germany). PCR was performed with Specific primers for bax, caspase-9, caspase-3, bcl-2 and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) as a house-keeping gene (Table 1). The primers were obtained from Sigma. All PCR reactions were performed using Maxima SYBR Green qPCR Master Mix (Bioline, London, UK) and were carried out using Agilent Mx3005P QPCR System (Agilent Technologies Co, CA, USA).
Each sample was analyzed in triplicate. Differences in gene expression between groups were calculated using the △△Ct (cycle threshold, Ct) method which were normalized against GAPDH and expressed as relative mRNA levels compared with controls.
A quantitative method was used to determine the percent of fragmentation of known amount of DNA into oligosomalsized fragments, where diphenylamine (DPA) attacks the bond between purines and deoxyribose liberating inorganic phosphate and provides the substrate (Schiff base). DNA fragmentation % in liver tissue was carried out as described elsewhere [12] with some modifications. The tissue ~(40 mg) was dissociated in 400 μl hypotonic lysis buffer (0.2 % triton X-100, 10 mMTris and 1mM EDTA) to obtain cell lysate. Cell lysate was incubated at 50ºC for 2.5 hours and centrifuged at 13,800 xg for 20 min. The supernatant containing the small fragments of DNA was separated immediately and the pellet dissolved in 350 μl TE (10 mMTrisHCl, 1mM EDTA, pH 8). Equal volume of 10% TCA was added to both supernatant and pellet, mixed well and incubated for 15 min at room temperature. After incubation, samples were centrifuged at 2,000 r.p.m for 10 min and the precipitate was resuspended in equal volume of 5% TCA then incubated at 100ºC for 10 min and cooled down at room temp. Two volumes of colorimetric reagent (0.088 M DPA, 98% v/v glacial acetic acid, 1.5% v/v sulfuric acid and 0.5% v/v 1.6% acetaldehyde solution) were added to one volume of the extracted DNA, kept at 37ºC overnight till blue color was developed. The developed blue color was quantified at 600 nm against blank (600 μl of 5%TCA).
Liver tissue was washed with isotonic saline then serial dilutions of ethanol were used for dehydration. Specimens were cleared in xylene and embedded in paraffin at 56ºC in hot air oven for 24 hours. Paraffin bee wax tissue blocks were prepared for sectioning at 4 μm thickness. The obtained tissue sections were deparaffinized and stained by hematoxylin and eosin for examination through the light microscope.
Statistical evaluation was conducted with Instat Program Graph-Pad (Software Inc, San Digeo, USA) version 3.6. Results were expressed as mean ± SEM. The results were analyzed for statistical significance by one way ANOVA followed by Tukey-Kramer multiple comparisons test. Values of P<0.05 were considered significant.
Results and Discussion
Thyrotoxicosis is a clinical manifestation of hyperactive thyroid gland. Large amounts of thyroid hormones produced from the gland induce abnormalities in liver [1]. Propylthiouracil, carbimazole and methimazole are used to treat hyperthyroidism but these drugs can also cause serious liver damage. The present study aimed to evaluate the prophylactic and therapeutic effects of safranal and selenite on liver damage induced by thyrotoxicosis.
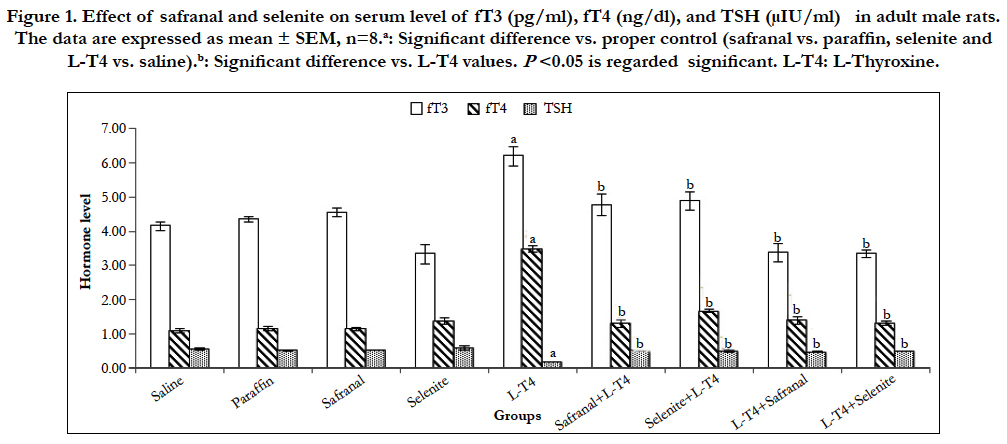
Treatment of rats with L-T4 resulted in significant (P<0.001) elevations (~49% and ~215%) in fT3 and fT4 levels, respectively as compared to the saline control (Figure 1). These high levels of serum fT3 decreased significantly with co-administration of either safranal or selenite with L-T4 by ~23% and 21%, respectively. Similarly, fT4 level decreased by 62% and 52%, respectively. There was a significant (P<0.001) reduction (~65%) in the serum TSH level in L-T4-treated rats as compared to the saline control. While safranal or selenite co-administration significantly elevated the TSH level ~164% and 158%, respectively. Post-treatment of hyperthyroid rats with either safranal or selenite caused significant reductions in the fT3 level by ~45%.Similarly, fT4 of post-treated animals decreased by ~61%. Treatment of hyperthyroid rats with either safranal or selenite caused a significant augmentation in the TSH level by ~143% and 152% when compared to L-T4 group, respectively (Figure 1). It is well known that H2O2 is needed by the thyroid as a cofactor in TH synthesis. GPx is the main antioxidant system for neutralizing cytotoxic H2O2 and its oxidative by-products. So, selenium may play an indirect role in the control of thyroid hormone synthesis because it is an integral part of the selenoenzyme thyroid peroxidase [13-15]. Moreover, the antioxidant activities of safranal and selenite that neutralize H2O2 would definitely reduce the THs production.
Figure 1. Effect of safranal and selenite on serum level of fT3 (pg/ml), fT4 (ng/dl), and TSH (μIU/ml) in adult male rats. The data are expressed as mean ± SEM, n=8.a: Significant difference vs. proper control (safranal vs. paraffin, selenite and L-T4 vs. saline).b: Significant difference vs. L-T4 values. P<0.05 is regarded significant. L-T4: L-Thyroxine.
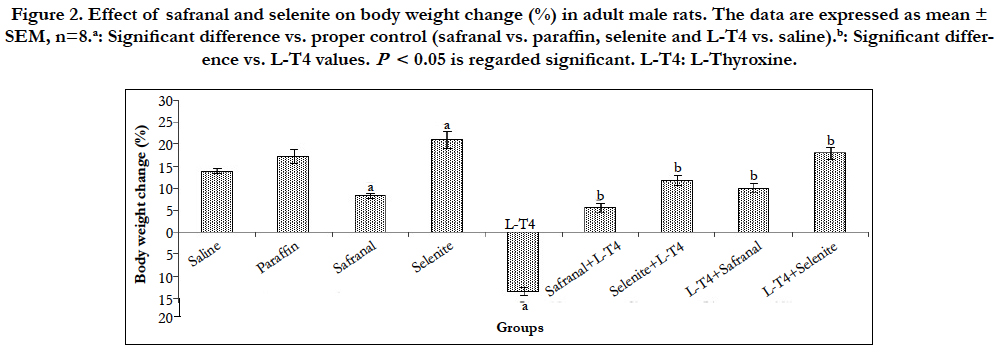
Animals treated with safranal showed a significant (P<0.001) decrease in body weight (~ -52.2 %) compared to the vehicle control; paraffin. Administration of selenite caused a significant (P<0.01) increase in the body weight change (~51.7 %) when compared to saline control. L-T4 caused a significant (P<0.001) reduction in the body weight (~ -196.0%) when compared to the saline control. Co-administration of safranal or selenite with L-T4 significantly (P<0.001) increased the body weight by ~142.0% and 187.9 %, respectively. Post-treatment of hyperthyroid rats with either safranal or selenite similarly caused a significant (P<0.001) increase in body weight by ~174.8 % and 234%, respectively (Figure 2). Many theories explain the body weight loss in hyperthyroid animals; the overwhelming catabolic activity of the elevated thyroid hormones (THs), the acceleration of basal metabolic rate, energy metabolism and protein wasting of the body represent the major functions of THs in several mammalian species, the depletion in fat stores and muscle protein by accelerated catabolism is another possibility [16], and oxidative stress produced by hyperthyroidism adversely affects adipocytes reducing the body fat and weight [17]. Safranal and selenite reversed this reduction in body weight and this could be attributed to their primary reducing effect on THs levels. The antioxidant effect of both safranal and selenite is another plausible mechanism.
Figure 2. Effect of safranal and selenite on body weight change (%) in adult male rats. The data are expressed as mean ± SEM, n=8.a: Significant difference vs. proper control (safranal vs. paraffin, selenite and L-T4 vs. saline).b: Significant difference vs. L-T4 values. P< 0.05 is regarded significant. L-T4: L-Thyroxine.
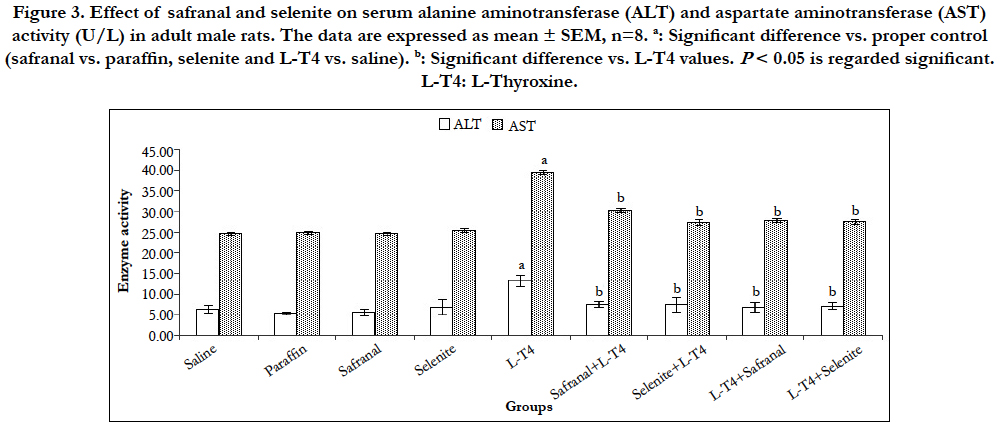
Statistical analysis of data in (Figure 3) revealed that L-T4 administration caused significant (P<0.001) elevations (~108% and 61%, respectively) in ALT and AST activities as compared to the saline control. This ALT increment was reduced by ~ 44% when L-T4 was co-administered with safranal or selenite. Treatment of hyperthyroid rats with either safranal or selenite caused a reduction in activity by ~49% and 46%, respectively (Figure 3). The coadministration of safranal or selenite with L-T4 to rats reduced AST activity by 23 % and 31%, respectively.The treatment of hyperthyroid rats with either safranal or selenite caused a similar decrease in AST activity by ~30% when compared to L-T4 group (Figure 3). Thyrotoxicosis is characterized by increased metabolic rate in liver. Blood circulation in the liver remains mainly steady but oxygen requirement is increased. In absence of any oxygen supplementation, the liver is adversely affected leading to tissue destruction and elevated levels of ALT and AST. However, the severe liver damage happened in area with lower oxygen demand [1]. Therefore, we believe that the oxidative stress, reduction in antioxidant ability, and cellular damage from LPO play a role in hepatic parenchyma damage and release of liver enzymes. The elevated ALT and AST activities after L-T4 were inhibited by either safranal or selenite administration. Safranal and selenite showed significant antioxidant and free-radical scavenging activities protecting the polyunsaturated fatty acids in cell membranes from damage and enhanced the regeneration of hepatic cells [18, 19]. This is evidenced from their effects on the terminal product of LPO; malondialdehyde (MDA).
Figure 3. Effect of safranal and selenite on serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activity (U/L) in adult male rats. The data are expressed as mean ± SEM, n=8. a: Significant difference vs. proper control (safranal vs. paraffin, selenite and L-T4 vs. saline). b: Significant difference vs. L-T4 values. P < 0.05 is regarded significant. L-T4: L-Thyroxine.
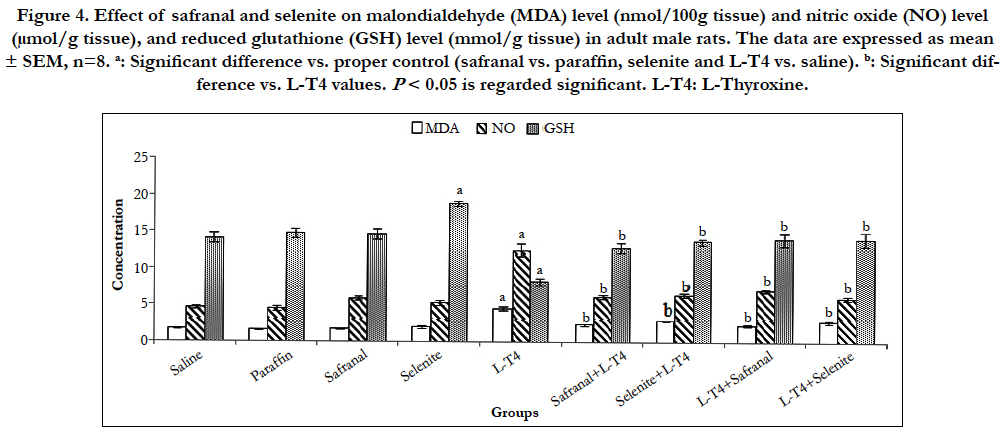
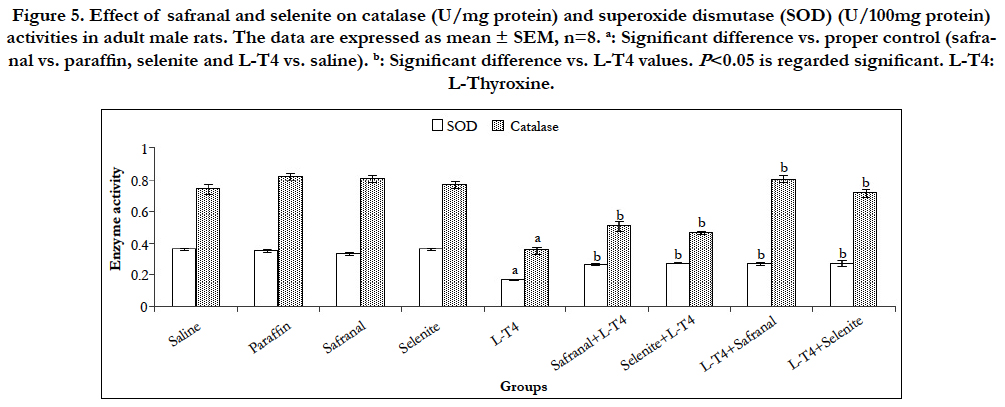
A highly significant (P<0.001) increase in the malondialdehyde (MDA) level was recorded after L-T4 administration (~171%) when compared to saline control. Whereas, co-administration of safranal or selenite with L-T4 lowered this MDA elevation by ~48% and 36%, respectively. Treatment of hyperthyroid rats with either safranal or selenite had a similar diminishing effect on the MDA level by ~50% and 38%, respectively (Figure 4). L-T4 also caused a significant (P<0.001) rise (~170%) in hepatic NO level as compared to saline control. Administration of safranal or selenite either with or after L-T4 reversed this increase significantly (P<0.001) by ~44-50% as compared to L-T4 treated animals (Figure 4). Selenite administration caused a significant (P<0.001) elevation ~33% in GSH level as compared to saline control. A significant (P<0.001) decrease (~42%) in hepatic GSH content following L-T4 administration was reported as compared to saline control. Co-administration of safranal or selenite with L-T4 augmented the GSH level by ~56% and 67%, respectively. Treatment of hyperthyroid rats with either safranal or selenite caused a similar augmentation in the GSH level by ~71% as compared to hyperthyroid animals (Figure 4). L-T4 induced a significant (P<0.001) reduction (~53%) in the catalase and SOD activities as compared to the saline control (Figure 5). Co-administration of safranal or selenite with L-T4 enhanced the catalase activity by ~44% and 32%, and SOD activity by ~57% and 61%, respectively. The treatment of hyperthyroid rats with either safranal or selenite similarly caused an increase in the catalase activity by ~128% and 103%, respectively and reversed the reduction in the SOD activity caused by L-T4 and enhanced the enzyme activity by ~58% and 60%, respectively (Figure 5). Hyperthyroidism increased the production of ROS, generation of hydroxyl radicals and LPO and resulted in increased MDA production through induction of calcium efflux in cytosol and imbalance of calcium homeostasis [3]. Thyroid hormones are known to increase mitochondrial respiration via changes in the number and activity of mitochondrial respiratory chain components leading to generation of superoxide radicals as a consequence of increase in cellular metabolism. These superoxide radicals in turn produce hydroxyl radicals which subsequently initiate LPO. Hyperthyroidism increased the rate of H2O2 release from liver mitochondria and peroxisomes which can easily diffuse into the cytosol [20]. Nitric oxide (NO) is produced through the transformation of L-arginine to L-citruline by a family of enzymes known as NO synthases (NOS). Thyroid hormone-induced calorigenesis involving enhanced liver O2 consumption rates led to progressive increments in the activity of hepatic NOS with a consequent high level of NO in rats [21]. The elevated NOS activity may be related to the increased number of liver macrophages induced by elevated T3 [22]. The changes in oxidative/antioxidants parameters were mitigated by safranal and selenite administration. Safranal was shown to inhibit the inducible nitric oxide synthase (iNOS), reduced peroxynitrite levels, and restored mitochondrial function.Safranal and selenite have been shown to have a hydroxyl radical scavenging activity and to elevate total sulfhydryl contents, antioxidant capacity and decrease MDA, NOS and hence NO levels [23]. Safranal and selenite inhibited LPO by inducing GSH, SOD and catalase reducing the free radicals formation and LPO which further improves membrane fluidity. SOD is an important defense system to convert superoxide radicals into H2O2 and catalase decomposes the resulting H2O2 to water and oxygen; thus, these enzymes together with GPx may contribute to the modulation of cellular redox milieu. Antioxidant enzymes are capable of stabilizing or deactivating free radicals before they attack cellular components. They act by reducing the energy of the free radicals or by giving up some of their electrons, thereby causing free radicals to become stable.
Figure 4. Effect of safranal and selenite on malondialdehyde (MDA) level (nmol/100g tissue) and nitric oxide (NO) level (μmol/g tissue), and reduced glutathione (GSH) level (mmol/g tissue) in adult male rats. The data are expressed as mean ± SEM, n=8. a: Significant difference vs. proper control (safranal vs. paraffin, selenite and L-T4 vs. saline). b: Significant difference vs. L-T4 values. P< 0.05 is regarded significant. L-T4: L-Thyroxine.
Figure 5. Effect of safranal and selenite on catalase (U/mg protein) and superoxide dismutase (SOD) (U/100mg protein) activities in adult male rats. The data are expressed as mean ± SEM, n=8. a: Significant difference vs. proper control (safranal vs. paraffin, selenite and L-T4 vs. saline). b: Significant difference vs. L-T4 values. P<0.05 is regarded significant. L-T4: L-Thyroxine.
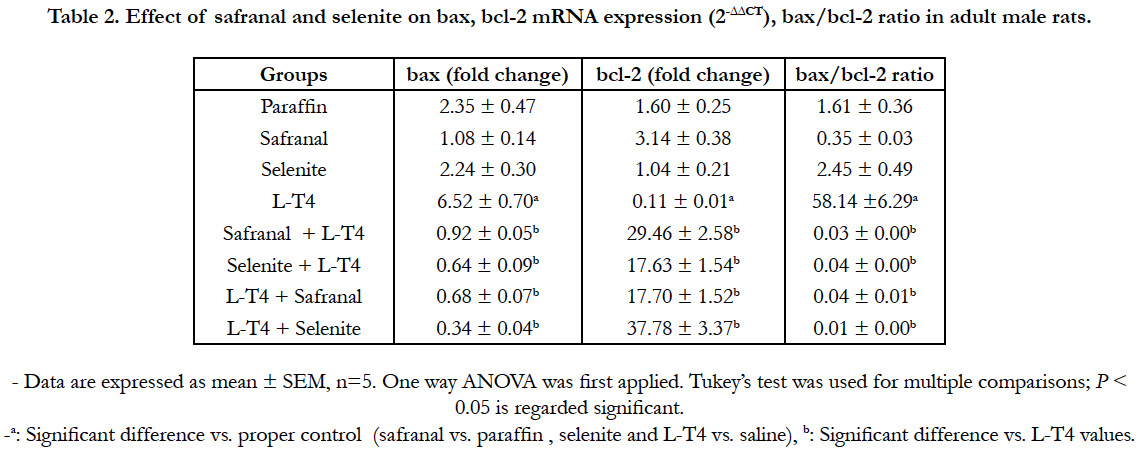
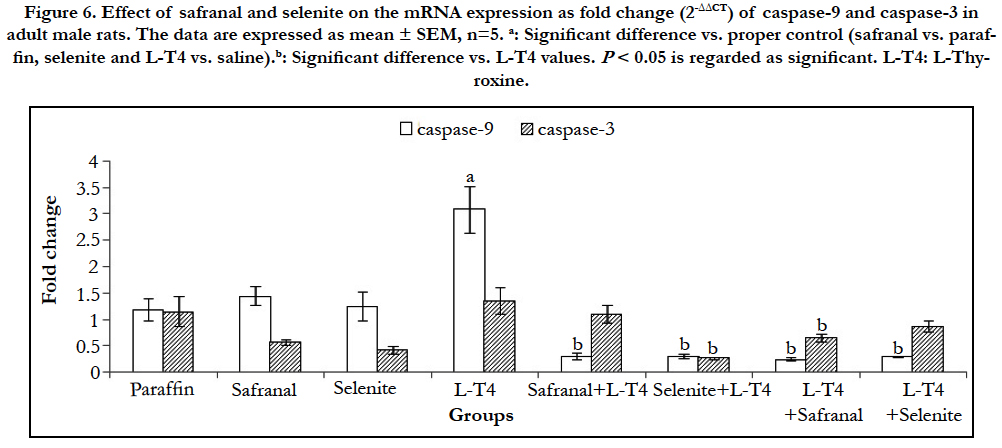
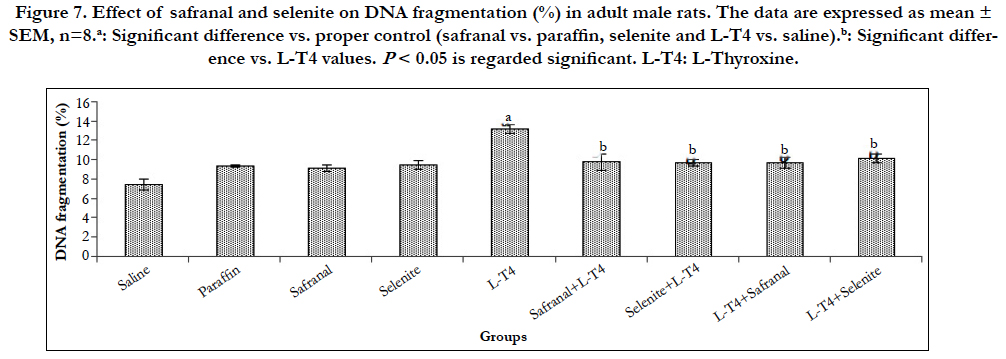
When the cells are damaged beyond the repair, cells go through apoptosis or necrosis depending on the severity of damage. A significant (P<0.001) upregulation in the mRNA expression of bax and caspase-9 in liver was observed in L-T4-treated rats by ~7 and 3 folds, respectively as compared to the saline control (Table 2 and Figure 6). However, co-administration of safranal or selenite with L-T4 downregulated the mRNA expression of bax to ~0.92 and 0.64 fold, respectively and caspase-9 to ~0.30 when compared to L-T4. Treatment of hyperthyroid rats with either safranal or selenite caused a similar downregulation in the mRNA expression of bax to ~0.68 and 0.34 fold, respectively (Table 2). A similar downregulation in the mRNA expression of caspase-9 to ~0.24 and 0.29 fold was shown on treating of hyperthyroid rats with either safranal or selenite, respectively (Figure 6). Treatment of rats with L-T4 resulted in a non-significant upregulation in the expression of caspase-3 mRNA in liver tissue by ~1.4 fold change as compared to saline control (Figure 6). Co-administration of selenite with L-T4 caused a significant (P<0.001) downregulation in caspase-3 mRNA expression to ~0.26 fold as compared to L-T4-treated animals. Treatment of hyperthyroid rats with either safranal or selenite caused a downregulation in the mRNA expression of caspase-3 to ~0.65 and ~0.87, respectively. However, only downregulation caused by safranal was statistically significant (P<0.05). The expression of hepatic bcl-2 mRNA was significantly (P<0.01) downregulated in L-T4 administered rats to ~0.11 fold as compared to saline control (Table 2). Co-administration of safranal or selenite with L-T4 resulted in significant upregulations in mRNA expression of bcl-2 by ~30 and 18 folds, respectively. Treatment of hyperthyroid rats with either safranal or selenite also caused significant upregulations in the mRNA expression of bcl-2 by ~18 and 38 folds, respectively. Administration of L-T4 caused a highly significant (P<0.001) upregulation ~58 fold in bax/bcl- 2 ratio when compared to saline control while co-administration of safranal or selenite with or after L-T4 significantly (P<0.001) downregulated this increment to ~ 0.01-0.04 folds. A highly significant (P<0.001) increment (~76 %) in the DNA fragmentation % in liver was recorded in L-T4 administered group as compared to saline control. This elevation was significantly reversed by administration of safranal or selenite either with or after L-T4 by 26% (Figure 7). The Bcl-2 family of proteins, which includes both pro-apoptotic genes (bax) and anti-apoptotic genes (bcl-2) members, is perhaps the most important regulator of the intrinsic apoptotic pathway. These proteins act at the mitochondria to integrate death and survival signals where the balance between pro- and anti-apoptotic members of the family as well as their interactions, determine whether the intrinsic pathway of apoptosis is initiated or not [24]. Caspase-9 is one of the initiator caspases and caspase-3 is one of the main effector caspases involved in apoptosis. Apoptosis increased in the liver of the thyrotoxic rats. Following an apoptotic stimulus; Bax undergoes conformational changes, integrates into the outer mitochondrial membrane, and oligomerizes causing membrane permeabilization [25]. The oxidative stress caused by hyperthyroidism can induce apoptosis by damaging mitochondrial DNA, inhibiting the mitochondrial respiratory chain transition, and increasing mitochondrial membrane permeability. The increased mitochondrial membrane hyperpolarization after exposure to H2O2 produced by hyperthyroidism initiated the collapse of mitochondrial membrane potential, mitochondrial translocation of Bax, and cytochrome c release [26]. The NO produced induces guanylatecyclase to produce cGMP from GTP. cGMP is responsible for cellular hyperpolarization due to the activation of the potassium channels [27]. T3 may act directly on mitochondria altering its structure and inducing permeability transition leading to the release of apoptogenic proteins and activation of caspases and apoptosis [28]. The current study revealed a significant downregulation in bax, caspase-3 and caspase- 9 mRNA expression with safranal or selenite administration. Safranal and selenite abrogate apoptosis by decreasing ROS and THs levels and enhancing the antioxidant cellular capacity. Safranal protects hepatocytes by inhibiting LPO and cell proliferation, inhibits apoptosis by increasing GSH, SOD and catalase during oxidative stress. In the current study, hepatic bcl-2 was significantly downregulated in thyrotoxic group. Bcl-2 inhibits apoptosis by preventing the release of the apoptosis-inducing factors and cytochrome c from the mitochondria [25]. The ratio of Bcl-2/ Bax regulates nuclei integrity and cell survival by controlling mitochondrial membrane permeability [25]. Decreased mitochondrial membrane stability and pore formation initiates the release of cytochrome c, formation of the apoptosome, and subsequent activation of caspase-9 [25]. THs inhibit Bcl-2 by dephosphorylation in mitochondria reducing the heterodimer Bcl-2/Bax elevating apoptosis [29]. The current results revealed a significant hepatic bcl-2 upregulation with safranal and selenite administration. Apoptosis is essential in getting rid of damaged cells. It is accompanied by biochemical features such as DNA fragmentation and degradation of specific cellular proteins as a result of a massive activation of a large number of intracellular proteases and endonucleases. In the present study, hepatic DNA fragmentation% was elevated in thyrotoxic rats. Elevation of caspases would lead to cleavage of different intracellular targets, which results in cell shrinkage, chromatin condensation, and DNA fragmentation [28]. The administration of safranal or selenite with L-T4 decreased the DNA fragmentation. This could be attributed to their antioxidant and free radical scavenging activities, elevation of DNA repair genes, stabilization of mitochondrial membrane and their effects on apoptotic/antiapoptotic signals mentioned before.
Table 2. Effect of safranal and selenite on bax, bcl-2 mRNA expression (2-ΔΔCT) and bax/bcl-2 ratio in adult male rats.
Figure 6. Effect of safranal and selenite on the mRNA expression as fold change (2-ΔΔCT) of caspase-9 and caspase-3 in adult male albino rats. The data are expressed as mean ± SEM, n=5. a: Significant difference vs. proper control (safranal vs. paraffin, selenite and L-T4 vs. saline).b: Significant difference vs. L-T4 values. P < 0.05 is regarded as significant. L-T4: L-Thyroxine.
Figure 7. Effect of safranal and selenite on DNA fragmentation (%) in adult male rats. The data are expressed as mean ± SEM, n=8.a: Significant difference vs. proper control (safranal vs. paraffin, selenite and L-T4 vs. saline).b: Significant difference vs. L-T4 values. P < 0.05 is regarded significant. L-T4: L-Thyroxine.
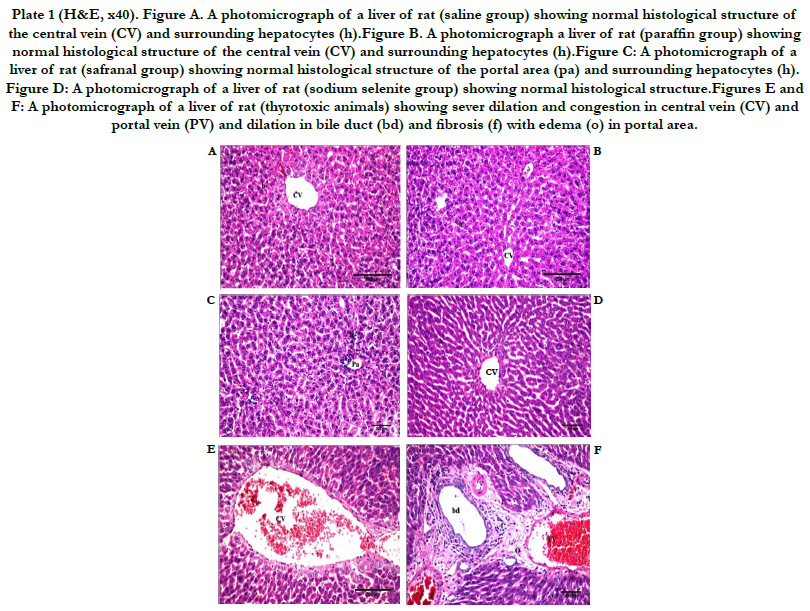
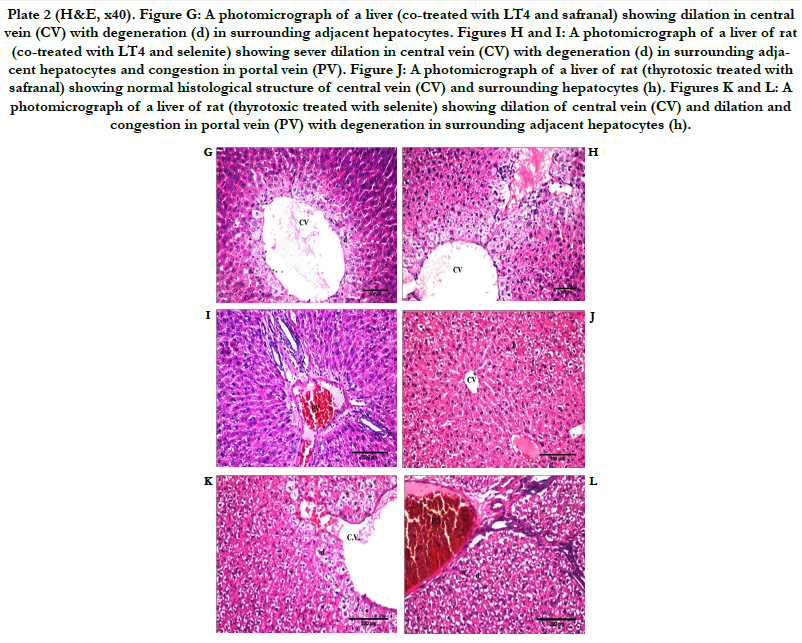
Normal architecture of liver tissue was observed in the saline, paraffin, safranal, and selenite treated groups. The hepatocytes are arranged in cords or plates, one or two cell thick, forming the normal liver cords radiating from the central vein (CV). The hepatic sinusoids are lined with flattened endothelial cells and few Kupffer cells. The nuclei of the later are spindle shape. The cytoplasm of the hepatic cells is pink in color with scattered basophilic granules. The nuclei of the hepatic cells are rounded in shape with granular chromatin material. Some hepatocytes are binucleated (Plate 1, Figures A-D). Histopathological examinations of liver section in tissue of animals treated with L-T4 for 21 days, revealed severe dilation and congestion in central and portal veins (PV). Dilation in bile duct (bd) and fibrosis (f) accompanied with edema (o) in portal area could also be seen (Plate 1, Figures E and F). Severe dilation in CV with degeneration in surrounding adjacent hepatocytes was observed in animals treated with safranal with L-T4 (Plate 2, Figure G). Liver tissue in animals treated with sodium selenite with L-T4 showed severe dilation in CV with degeneration in surrounding adjacent hepatocytes with congestion in PV (Plate 2, Figures H and I), however, normal histological structure of central vein (CV) and surrounding hepatocytes (h) was demonstrated when L-T4 administered was followed by administration of safranal (Plate 2, Figure J) in agreement with previous reports [30, 31]. However, liver tissue in animals treated with sodium selenite after L-T4 administration, showed dilation of CV with degeneration in surrounding adjacent hepatocytes, dilation and congestion in PV (Plate 2, Figures K and L). The histopathology study of liver confirms the biochemical findings of the present investigation.
Plate 1 (H&E, x40). Figure A. A photomicrograph of a liver of rat (saline group) showing normal histological structure of the central vein (CV) and surrounding hepatocytes (h).Figure B. A photomicrograph a liver of rat (paraffin group) showing normal histological structure of the central vein (CV) and surrounding hepatocytes (h).Figure C: A photomicrograph of a liver of rat (safranal group) showing normal histological structure of the portal area (pa) and surrounding hepatocytes (h). Figure D: A photomicrograph of a liver of rat (sodium selenite group) showing normal histological structure.Figures E and F: A photomicrograph of a liver of rat (thyrotoxic animals) showing sever dilation and congestion in central vein (CV) and portal vein (PV) and dilation in bile duct (bd) and fibrosis (f) with edema (o) in portal area.
Plate 2 (H&E, x40). Figure G: A photomicrograph of a liver (co-treated with LT4 and safranal) showing dilation in central vein (CV) with degeneration (d) in surrounding adjacent hepatocytes. Figures H and I: A photomicrograph of a liver of rat (co-treated with LT4 and selenite) showing sever dilation in central vein (CV) with degeneration (d) in surrounding adjacent hepatocytes and congestion in portal vein (PV). Figure J: A photomicrograph of a liver of rat (thyrotoxic treated with safranal) showing normal histological structure of central vein (CV) and surrounding hepatocytes (h). Figures K and L: A photomicrograph of a liver of rat (thyrotoxic treated with selenite) showing dilation of central vein (CV) and dilation and congestion in portal vein (PV) with degeneration in surrounding adjacent hepatocytes (h).
Conclusion
The modulatory effects of safranal and selenite against the deleterious liver injury induced by thyrotoxicosis are mediated through bolstering the endogenous antioxidant defense systems. The mitigation offered by safranal was in many instances better than that shown after selenite administration in parameters such as body weight, MDA, and catalase. Treating the hyperthyroid animals with safranal or selenite for three weeks gave better palliating results than those reported for the protection regimen. This can be manifested in body weight, T3 and GSH levels, in addition to catalase activity and was also confirmed by the histopathological study. We understand the limitations of the animal model of hyperthyroidism used in this study and recommend further investigations in other animal models but this does not refute the adjunctive salutary effects of safranal and selenite on liver damage induced by hyperthyroidism in the current study. Further investigation is also needed to identify the mechanism(s) by which these two agents reduced the thyroid hormones.
References
- Khemichian S, Fong T-L (2011) Hepatic dysfunction in hyperthyroidism. Gastroenterol Hepatol. 7(5): 337-339.
- Choudhary AM, Roberts I (1999) Thyroid storm presenting with liver failure. J Clin Gastroenterol. 29(4): 318-321.
- Assaei R, Zal F, Mostafavi-Pour Z, Dabbaghmanesh MH, Geramizadeh B, et al., (2014) Hepatoprotective effect of Satureja khuzestanica essential oil and vitamin E in experimental hyperthyroid rats: evidence for role of antioxidant effect. Irani J Med Sci. 39(5): 459-466.
- Zhang S-P, Huang J-N, Jin N, Wang X-L, Jin C-C (2017) Safranal inhibits the migration and invasion of human oral squamous cell carcinoma cells by overcoming epithelial-mesenchymal transition. Biomed Res. 28(2): 817-821.
- Ognjanović B, Markovic S, Pavlovic S, Zikic R, Stajn A, et al., (2008) Effect of chronic cadmium exposure on antioxidant defense system in some tissues of rats: protective effect of selenium. Physiol Res. 57(3): 403-411.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J biol Chem. 193(1): 265-275.
- Uchiyama M, Mihara M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 86(1): 271-278.
- Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 5(1): 62-71.
- Van Doorn R, Leijdekkers C-M, Henderson PT (1978) Synergistic effects of phorone on the hepatotoxicity of bromobenzene and paracetamol in mice. Toxicol. 11(3): 225-233.
- Nandi A, Chatterjee I (1988) Assay of superoxide dismutase activity in animal tissues. J Biosci. 13(3): 305-315.
- Aebi H (1984) Catalase in vitro. Methods Enzymol. 105: 121-126.
- Perandones C, Illera V, Peckham D, Stunz L, Ashman R (1993) Regulation of apoptosis in vitro in mature murine spleen T cells. J Immunol. 151(7): 3521-3529.
- Hawkes WC, Keim NL (2003) Dietary selenium intake modulates thyroid hormone and energy metabolism in men. J Nutr. 133(11): 3443-3448.
- Bacić Vrca V, Skreb F, Cepelak I, Mayer L (2004) Supplementation with antioxidants in the treatment of Graves' disease: the effect on the extracellular antioxidative parameters. Acta pharmaceutica. 54(2): 79-89.
- Choksi NY, Jahnke GD, St Hilaire C, Shelby M (2003) Role of thyroid hormones in human and laboratory animal reproductive health. Birth Defects Research Part B: Develop Reprod Toxicol. 68(6): 479-491.
- Chakrabarti S, Guria S, Samanta I, Das M (2007) Thyroid dysfunction modulates glucoregulatory mechanism in rat. Ind J Exp Biol. 45(6): 549- 553.
- Kim S-M, Kim S-C, Chung I-K, Cheon W-H, Ku S-K (2012) Antioxidant and protective effects of Bupleurum falcatum on the L-thyroxine-induced hyperthyroidism in rats. Evid Based Complement Alternat Med. 2012: 1-12.
- Othman AI, El-Missiry MA (1998) Role of selenium against lead toxicity in male rats. J Biochem Mol Toxicol. 12(6): 345-349.
- Assimopoulou A, Sinakos Z, Papageorgiou V (2005) Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother Res. 19(11): 997-1000.
- Venditti P, Di Meo S (2006) Thyroid hormone-induced oxidative stress. Cell Mol Life Sci Cell Mol Life Sci. 63(4): 414-434.
- Fernández V, Cornejo P, Tapia G, Videla LA (1997) Influence of hyperthyroidism on the activity of liver nitric oxide synthase in the rat. Nitric Oxide. 1(6): 463-468.
- Tapia G, Pepper I, Smok G, Videla LA (1997) Kupffer cell function in thyroid hormone-induced liver oxidative stress in the rat. Free Radical Res. 26(3): 267-279.
- Bukhari SI, Pattnaik B, Rayees S, Kaul S, Dhar MK (2015) Safranal of Crocus sativus L. inhibits inducible nitric oxide synthase and attenuates asthma in a mouse model of asthma. Phytother Res. 29(4): 617-627.
- Guicciardi M, Gores GJ (2005) Apoptosis: a mechanism of acute and chronic liver injury. Gut. 54(7): 1024-1033.
- Nechushtan A, Smith CL, Lamensdorf I, Yoon S-H, Youle RJ (2001) Bax and Bak coalesce into novel mitochondria-associated clusters during apoptosis. J Cell Biol. 153(6): 1265-1276.
- Cui H, Kong Y, Zhang H (2012) Oxidative stress, mitochondrial dysfunction, and aging. J Signal Trans. 2012: 1-13.
- Raddino R, Caretta G, Teli M, Bonadei I, Robba D, et al., (2007) Nitric oxide and cardiovascular risk factors. Heart Int 3(1): 18-26.
- Upadhyay G, Singh R, Kumar A, Kumar S, Kapoor A, et al., (2004) Severe hyperthyroidism induces mitochondria‐mediated apoptosis in rat liver. Hepatol. 39(4): 1120-1130.
- Yehuda-Shnaidman E, Kalderon B, Azazmeh N, Bar-Tana J (2010) Gating of the mitochondrial permeability transition pore by thyroid hormone. The FASEB J. 24(1): 93-104.
- Ozkececi ZT, Gonul Y, Yuksel Y, Karavelioglu A, Tunay K, et al., (2016) Investigation of the effect of safranal and crocin pre-treatment on hepatic injury induced by infrarenal aortic occlusion. Biomed Pharmacother. 83: 160-166.
- Ozardal I, Bitiren M, Karakılçık AZ, Zerin M, Aksoy N, et al., (2004) Effects of selenium on histopathological and enzymatic changes in experimental liver injury of rats. Exp Toxicol Pathol. 56(1): 59-64.