Effects of Ranitidine on Insulin and Lime - Induced Gastric Secretion in Albinowistar Rats
Nwaichi, E. O.1*, Gwotmut, M. D.2 and Ossai, J.3
1 Department of Biochemistry, University of Port Harcourt, P. M. B. 5323 Port Harcourt, Rivers State, Nigeria.
2 Department of Physiology, University of Port Harcourt, P. M. B. 5323 Port Harcourt, Rivers State, Nigeria.
*Corresponding Author
Nwaichi E.O,
Department of Biochemistry,
University of Port Harcourt,
P. M. B. 5323 Port Harcourt,
Rivers State, Nigeria.
E-mail: nodullm@yahoo.com.
Article Type: Research Article
Received: January 11, 2013; Accepted: January 25, 2013; Published: January 31, 2013
Citation: Nwaichi, E. O., Gwotmut, M. D and Ossai, J. (2013) Effects of Ranitidine on Insulin and Lime - Induced Gastric Secretion in AlbinowistarRats. Int J Clin Pharmacol Toxicol. 2(1), 38-44. doi: dx.doi.org/10.19070/2167-910X-130008
Copyright: Nwaichi, E.O©2013. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Purpose: To study the possible effect (s) of a relative H2-receptor blocker, ranitidine on lime and insulin-induced gastric secretion in male and female albino rats.
Methods: The rats were divided into 3 groups of lime juice, insulin and control in triplicates after 24hr starvation to empty the stomachand were canulated (oesophageal, tracheal and gastric) using Gosh and Schild method. Using N saline, the acid content of the effluentwas recorded. The 1st group of rats was perfused with lime solution (25% v/v, 50%v/v, 75% v/v and 95% v/v) and was used to modify the secreto-ry rates of the parietal cells and the stomach effluent was collected 3 times in 30 minutes. In same man -ner, the 2nd group was perfused with 40 IU/ kg insulin. The 3rd group (control) had no lime nor insulin. Ranitidine was administered (2.5ml ) intramuscularly and the results noted.
Results: The mean basal secretion significantly (P≤ 0.05) increased from 22.82 ±4.6mMol/L/hr to 52.94 ±10.23mMol/L/hr, while 2.5ml ranitidine (Zantac)injected intramuscularly decreased the basal stimula-tion from 52.94 ±10.23mMol/L/hr to34.77 ± 5.09 mMol/L/hr. Insulin (40 IU/kg) was administered in-travenously to the 2nd group of rats, and the mean basal secretion increased from 8.01±0.75 mMol/L/hr to10.00±0.71 mMol/L/hr. Ranitidine was administered intramuscularly and that caused a significant decrease in the insulin stimulation from 10.00±0.71 mMol/L/hr to 8.01±0.75 mMol/L/hr.
Conclusion: Results obtained showed marked higher gastric secretion in females than in males, although stead increase was observed for both insulin and lime inducement.Generally, results obtained from this study indicated, a statistically significant decrease in both lime juice and insulin-induced gastric secretion by Ranitidine, and hencesubmits Ranitidine as a possible potent drug for peptic ulcer treatment.
2.Introduction
2.1 Cephalic Phase
2.2 Gastric Phase
2.3 Intestinal Phase
2.4 Insulin
2.5 Histamine
2.6 Peptic Ulcer
2.7 Ranitidine (Zantac)
2.8 Lime (Citrus Aurantifolia):
3.Results
3.1 Lime-Induced Gastric Secretion
3.2 Insulin-Induced Gastric Acid Secretion
4.Discussion
4.1 Lime-induced gastric secretion in female Wistar albino rats
4.2 Lime-induced gastric secretion in male Wistar albino rats
4.3 Insulin-induced gastric secretion in female Wistar albino rats
4.4 Insulin-induced gastric secretion in male Wistar albino rats
5.Conclusion
6.References
Keywords
Agonists, Gastric acid secretion, Insulin, Lime, Inhibition.
Introduction
One of the functions of the stomach is the secretion of gastric acid by oxyntic cell. Gastric juice secreted by the gastric gland of the stomach contains a number of gastric enzymes and acid, which were identified in 1824 to be hydrochloric acid.The acid plays a key role in digestion of potassium chloride and sodium chloride (Sembulingam and Sembulingam, 2010) and in digestion of proteins, by activating digestive en -zymes, and making ingested proteins unravel so that digestive enzymes can break down the long chains of amino acids (Ward et al., 1963).The ultimate source of H+ is water. The ultimate source of chloride ion is the sodium chloride found in blood. The reaction (overall) can best be summarized in the reaction below:
For each mole of HCl produced, a mole of Na2 CO3 is formed. The carbon IV oxide used for the reaction is derived from the parietal cell metabolism itself and also from the cellular metabolism of other tissues in the body and for every H+ produced, a bicarbonate ion is released into the intestinal fluid and ultimately into the blood. This explains the so called alkaline tide seen in the urine during digestion (Soll and Read, 1991).
On stimulation, the morphological structure of the parietal cells have been found to change during secretion of gastric acid and this has been found to be essential component of gastric acid secretory process (Wormsley, 1968; Yao and Forte, 2003).
The synthesis and secretion of hydrochloric acid by oxyntic cells have been studied extensively; H+ are secreted to obtain pH of 1.0 when intracellular pH is 7.1-7.2, achieving a 2 million fold concentration gra -dient. Carbonic anhydrase catalyze the formation of bicarbonate and H+ from carbon IV oxide and water (Davenport, 1939). An enzyme (H+/K± ATpase), locat -ed on the surface of the oxyntic cell lumen, secretes H+ across the apical membrane in exchange for potas-sium ion, by a separate pathway. Potassium ion and chloride ion are secreted into the canaliculi space, down their concentration gradient with water follow-ing the electrochemical and osmotic balance that is maintained across the apical membrane. Secretion of this large amount of acid into the lumen requires that an equivalent amount of base be secreted from the basolateral surface of the oxyntic cell to maintain the intracellular pH in the physiological range. Chloride ons are exchanged for hydroxyl ion (OH-) concentra -tion. The Na+/K+ - ATpase located on the basolateral membrane maintains the higher intracellular potas -sium ion concentration (Vasudevan et al., 2007)
A variety of substances have been found to support acid secretion including glucose and fatty acids (Kon -turek et al., 1977; Roekpe et al, 2006). New classes of antisecretory drugs, the substituted benzimaclole appear to act by inhibition of this H+/K+ ATpase. Most recent data suggest that acid secretion at the canalicular membrane may be regulated by the potassium chloride permeability of the canaliculi membrane. Potassium chloride permeability would increase at the transition to stimulated state to provide K+ a luminal face of the H+/K+ ATPase for exchange (Geibel, 2005).
Acid secretion is commonly divided into 3 phases. Viz:i) Cephalic Phase, ii) Gastric Phase and iii) Intestinal phase.
Only a small proportion of gastrin is released dur-ing this phase, perhaps in part because, the fast-ing stomach is acidic and H+ inhibits gastrin re-lease. There could be abundant secretion of gastric juice even though there is no food in the stomach. This is readily demonstrated by sham feeding (Sembulingam and Sembulingam, 2010).
As food and fluids enters the stomach, the buffering effect of the content raises the pH, removing the in -hibitory effect of the low pH on gastric release; the effect is abolished by acidification of gastric content. Since secretion is also suppressed by atropine, and H2 antagonists, it is probably not due to direct effect on oxyntic cells. As food enters into the stomach, two factors are known to be operative; which are disten -sion of the stomach and stimulation by chemical mediators (Yang, 2002).
The secretion is mediated via release in the intestine of an unknown peptide, enteroxyntin, the stimulus for its release appears to be the presence of peptides and some amino acids. The latent period of the intestinal phase is two to three hours, but the secretion when initiated may last an average of 6 hours. This phase is characterized by less activity than the other two phases. The following have been observed to execute gastric secretion when placed in the small intestine, water, extractive substances of meat, products of protein digestion, milk, alcohol, histamine, saponin, epinephrine, 0.1N HCl, 10% glycine solution and magnesium sulfate (Troidl et al., 1975).
The chemical mediators of gastric secretion are clearly acetylcholine (a secreting neuron which innervates the gastric gland, can directly activate parietal cells and also the antral G-cells which release the hormone gastrin terminals can directly activate parietal cells and also the antral G-cells which release the hormone gastrin), gastrin (A peptide hormone that caused a strong stimulation of gastric acid secretion, released from the G-cells of the pyloric antrum and the two principally active forms of gastrin are small gastrin (G-17) and big gastrin (G-34)) , and histamine (Uvans, 1969).
Insulin was named by Demeyer in 1907 and obtained or isolated by Banting and Best in 1922 in Madeods Laboratory. It is a peptide hormone secreted by the beta cells of the islets of Langerhans, found in the pancreas. The hypoglycemia produced by insulin stimulates the sympathetic as well as parasympathetic centers. Stimulation of the latter causes the signals to be sent through the vagus nerves to cause gastric acid secretion and pepsin secretion (Schubert and Peura, 2008).
The existence of histamine oraminoethylimidazole was demonstrated in 1910 by Sir Henry Deland Barge while working on ergot alkaloids. It was until 1927 that Best, Dale, Dudely and Thorpe isolated hista -mine from fresh sample of lungs and live, thereby es -tablishing beyond doubt that histamine was a natural constituent of the body (Soll, 1978). Paton reported that histamine was present in all parts of the body, except bones and cartilages. All animal species have relatively high level of histamine in the glandular region of the stomach mucosa, where acid is secreted, and that there is a species difference on the distribution of histamine in the gastric mucosa, example in the rats, histamine is distributed in the entrerochro -maffin cells while in man, it is found in the mast cells.
Histamine has two types of receptors located throughout the body, which when occupied by histamine or one of its analogues produces different effects. Some of these effects such as bronchroconstriction and contraction of gut are mediated by the H1 receptors (Sandrik and Waldum, 1991) and are readily blocked by the classical antihistamine such as mepyramine and pyrilamine. Other effect such as gastric acid secretion is completely refractory of such antagonists and involves the activation of H2 receptor and is susceptible to the newly developed H2 antagonists.
Highly effective H2antagonist ,Burmamide retained the imidazole ring of histamine believed to be im -portant for receptor recognition, but possessed a much bulkier side chain. Examples of such drugs are metiamide, mizatidine, and oxmetidine, cimetidine and famotidine (Samuelson and Hinkle, 2003). It was found in Glaxo laboratory, that activity was retained in analogues of imidazole series when the imidazole moiety was replaced by an aminoethyl aromatic system. This gave rise to the compound ranitidine . Biochemical regulation of this secretion involves central signals conveyed by the vagus nerve and local mechanism mediated by cholinergic and peptidergic fibers of the gastric wall, as well as amine or pep -tides secreting cells located in the fundic and antral epithelia (Uvans, 1969). The blockade of this recep -tor by specific antagonist results in a surmountable inhibition of gastric acid secretion. The blockade of H+ K±ATpase is an alternative means of inhibition, this can be achieved by series of benzimide derivatives iondazopyrimidine which specifically accumulates in he parietal cell secretory canaliculus and covalently binds to ATPase inhibitory site (Roekpe et al., 2006; Code, 1949). It has longer than histamine antagonist. Therefore, H+ K± ATPase inhibitors are of special interest in the treatment of acid-related diseases such as gastric and duodenal ulcer, as well as es -ophagitis reflux (Oliver, 1960; Feldman et al., 1998).
This is a breach in the mucosa of the digestive tractproduced by digestion of the mucosa by pepsin and acid. This may occur when pepsin and acid are present in abnormally high conc. or when some other mechanism reduces the normal protective mechanisms of the mucosa.The results from Lee et al. (2001) correlated with peptic ulceration and evidence has shown that over secretion of gastric acid and gastric juice can be corrosive to the gastric mucosa and can consequently lead the development of peptic ulceration. Emotional and physical stress, hypermotility, vascular spasm and thrombosis, infection, allergy and reduced and altered mucosa production are some of the factors that could cause ulceration of the mucosa.
Accordingly, an improved H2antagonist has been sought and results now described, lead us to believe that ranitidine, N-2, 5 (dimethyl amino), methyl-2-nitro-1, 1-ethenediamene is such a drug.Ranitidine was synthesized by members of the chemistry division, Glaxo Group research limited. Ranitidine is a new specific histamine antagonist which differ chemically from other histamine receptor blockers in having a furan rather than an imidazole or thia -zole ring structure. Comparison with cimetidine has shown ranitidine to be four to ten times more potent antagonist. However, cimetidine has been shown to be a specific histamine H2receptor antagonist despite the substitution of the imidazole by a furan ring. Ranitidine like cimetidine is effective clinically in the short term management of duodenal ulcer.
Ranitidine inhibits the acid and pepsin secretion which is induced by various stimuli (histamine, lime, pentagastrin, meal). The inhibitory effect on histamine is an expected of a competitive nature, that on pentagastrin is non competitive. Depending on the test system used, ranitidine is up to ten times more potent than cimetidine on molar basis (Prichard et al. 1986).Ranitidine does not seem to affect cardiovascular function in a close range that can be useful. In duodenal ulcer patients, doses of ranitidine assumed to be therapeutically effective, does not change the concentration of follicle stimulating hormone, luteinizing hormone, prolactin, oestradiol and testosterone in the plasma, thus suggesting that the drug does not affect the endocrine system.
Lime is a term referring to citrus fruit which is typically round, green to yellow in color (Hulma, 1971), 3-6cm in diameter and containing sour and acidic pulp. Lime is a good source of vitamin and is often used to accent the flavors of foods and beverages. Lime juice may be squeezed from lime and is used as limeade, and as an ingredient in many cocktails. When the skin is exposed to ultraviolet light, after lime juice contact, a reaction known as phytophotodermatitis can occur, this can cause darkening of skin, swelling or blister-ing. The agent responsible for this is Psoralen. Lime juice and its oil are very beneficial for skin when consumed orally or applied externally. It rejuvenates the skin, keeps it shining protects it from infections and reduces body odor due to large amounts of Vitamin C and flavonoids both of which are antioxidants. When applied externally onto the skin, lime acids slough off dead cells (Kokwaro, 2009).
Lime also helps in areas where circulation is a problem; such is the case with allulite. The therapeutic properties of lime oil in skin -care are antiseptic, antiviral, disinfectant bactericid -al, astringent, restorative and tonic.Trivett and Meyer (1971)reported that citric acid is one of the most common acids in lime and is present in small quantity (8-9%). The quantity of the acid varies with season, ripeness and variety. The juice predominates in citric acid than the peel, while the peel of lime fruit has a higher content of oxalic acid than citric and malonic acid (Hulma, 1971).He also reported that chemical changes during maturation were broadly reverse of those of orange and grape juice.This study seeks to evaluate the following:
i) effects of insulin and lime juice on gastric acid secretion in the Wistar albino rats.
ii) effects of ranitidine on insulin and lime induced acid secretion in Wistar albino rat.
Materials And Methods (Gosh And Schild Methods)
Albino Wistar rat were sourced from Animal house of the Biochemistry department of the University of Port Harcourt Nigeria. Each rat (200-250g) was starved for 24 hours/overnight in order to empty the stomach by the following day. Each rat was then weighed and divided into 3 groups of 5 each for males, females and control). Anesthesia (Urethane) was administered (0.6mg per 100gram body weight) intraperitionally along the linea Alba. Each rat used, slept, within five minutes of the administration of the anesthesia. However, if the dose was not enough, an extra 0.1-0.2ml was given.
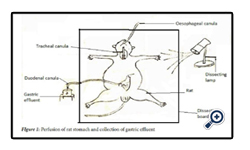
The rat was then pinned to the dissecting board and the dissecting light turned on (Fig 12). The skin around the neck of the rat was then removed, along the midline to expose the thyroid gland which was then separated by blunt dissection to expose the tra -chea (Fig 2.0). The trachea was then freed from the surrounding tissues. A sea - porter was passed under the trachea and a tracheostomy was performed by making a semi-transection of the upper rings of the trachea. A tracheal canula (dipped in Normal saline) was then inserted and it was then tied into position using needle and thread avoiding the vagus nerve. This allowed the animal to breath freely. However, the canula was monitored to detect mucus accumu -lation due to irritation. If present, it was removed with a hypodermic needle. The wound was then cov-ered with cotton wool damped with normal saline. The skin over the linea Alba was then lifted up and out to expose the abdominal muscle. The abdomi -nal muscle was then incised along the linea alba but the incision was then brought towards the left hy-pochondria. The stomach was then brought out by fingers dipped in normal saline. After, a cut was then made on the anterior surface not too far from the pylorus. A gastric canular was introduced into the stomach and tied into position and switched back to keep temperature warm.
An esophageal tube connected to the perfusion bottle was then introduced into the stomach via the mouth and the esophagus. Normal saline kept
at constant temperature of 370C was then allowed to flow through the tube from the perfusion bottle and the residual contents of the stomach were com -pletely flushed out and the effluent discarded. When the stomach effluent was found to be free of food debris, the flow rate was regulated to give a volume of 10ml+ 4ml/minute at time interval of 10 minutes. More of the saline thus, had to be added to the bot-tle when needed.
After the flow rate had been adjusted, 3 basal collec -tion of the stomach effluent were titrated against so -dium hydroxide to find the concentration of the acid content of the effluent. The saline was removed fromthe perfusion bottle and quickly replaced with differ-ent contents of lime juice (25% v/v, 50% v/v, 75% v/v, and 95% v/v) and 3 basal collections were made at 10ml + 4mls/minutes at time interval of 10minutes.
Each basal collection was titrated against sodium hydroxide and the results were noted (for n=5) and mean values taken accordingly.
Ranitidine (Zantal, 50mg) was administered (2.5ml)intramuscularly and 3 check 4 repetition basal collec -tion was made at time interval of 10minutes.On eachrat, lime of 25% v/v, 50% v/v, 75% v/v and 95% v/v were used to flush the stomach and 3 basal collec -tions were titrated against sodium hydroxide and the results were noted.
The second group of rats was anaesthetized, dissect-ed and canulated. Normal saline in the perfusion bot-tle was used to flush the stomach via the mouth to free the stomach from food and other debris. 3 basal collections were made and titrated against sodium hydroxide, for each rate. Insulin (0.5ml / body kg)was administered intramuscularly (1.m). The stomach effluent was then collected 3 times in 30 minutes. Ranitidine was then administered intramuscularly at a dose of 25ml/body weight, 3 basal collections were made and titrated against sodium hydroxide as
described above. Statistical analysis was done using Student’s t – test to essentially compare the means of paired samples.
The results obtained for lime-induced gastric secre -tion in albino wistar rats (3males and 2 females) with lime content of 25% v/v, 50% v/v, 75% v/v and 95% v/v) within 30mins are as given in tables 3.1 and 3.2. Stimulation and inhibition are also presented.
The results obtained for insulin-induced gastric secretion in 5 wistar albino rats (male and female groups) is as given below. Stimulation and inhibition are also presented.
Female albino Wistar rats were used to test the ef-fect of relative new H2-receptior blocker, ranitidine.
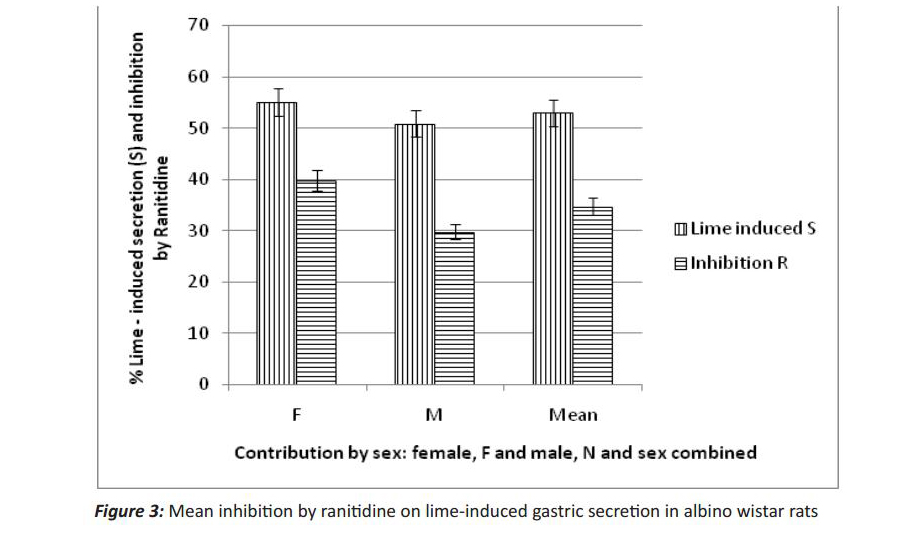
The mean basal secretion was 17.30±9.2mMol/L/hr and increased to 54.86±9.2mMol/L/hr after perfu -sion with lime of different concentration (25%, 50%, 75% and 95%) (Fig 2). This increase in acid secre-tion caused by lime juice was statistically significant on applying the student t-test and did not occur by chance. Administrations of the test drug, ranitidine (2.25mg/kg), after 30 minutes, caused a decrease in gastric secretion from 54.86±9.2mMol/L/hr to 29.78±7.67mMol/L/hr (Fig 3). Thus, the decrease in acid secretion caused by ranitidine was found to be significant and thus did not occur by chance.
Male albino Wistar rats gave a mean basal of 28.33±11.14 mMol/L/hr which increased to 51.71±10.20 (Table 3.2) with lime. Administration of the test drug decreased the stimulation by lime juice to 29.78±7.67 (graph 2). This significant increase by lime juice wasinhibited by the test drug and did not occur by chance but was marked. Higher gastric secretion in females was observed (Figs 2 and 4) and secretion increased with weight (data not shown).
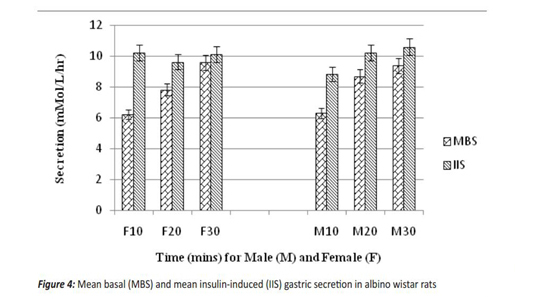
Wistar albino rats Female albino wistar rats were induced with 0.5ml insulin (Actrapid) and the basal mean se -cretion increased from 8.70±0.62mMol/L/hr to 10.10±1.01mMol/L/hr. (Fig 4).Administration of the test drug inhibited secretion from 10.10±1.01 mMol/L/hr to 8.70±0.62 mMol/L/hr.(Fig 5)Student’s t – test showed that these did not occur by chance but was significant.
These rats were induced with insulin (0.5ml) which caused an increase from 7.89 1.10 mMol/L/hr mean basal secretion to 9.90±0.37 mMol/L/hr (Fig 4). This decreased to 7.96±0.37 mMol/L/hr (Fig 5),on admin -istration of the test drug. These increase and decrease did not occur by chance.The great increase in acid secretion is believed to be strength dependent. The probable mechanism by which lime caused an increase in gastric secre-tion is through the gastric mechanism and by direct effect on the parental cells(Kay, 1953).Insulin on the other hand caused an average increase in acid secre-tion. Insulin has been found to stimulate acid secre-tion through its effect on the vagus nerve. Insulin-induced hypoglycemia stimulates the vagal nerve which in turn stimulates acid secretion (Forte and Zhu, 2010; Aihara et al., 2003).
Ranitidine which is a H2-receptor blocker, caused an inhibition due to its action on the H2-receptor, which mediates secretion, and its action is said to be specif-ic.From this experiment, ranitidine exerted a greater effect on lime-induced gastric secretion than insu -lin. This explains that histamine is not a final com -mon mediator for the action of other stimulation, but a potentiate (Bonfils et al., 1979;Pritchard et al., 1986). As previous studies have shown that, inhibi -tion is not as a result of reduced mucosal blood flow (Prema and Smbulingam, 2010; Troidl et al., 1975), it wasobserved that the antisecretory activity of ran-itidine stems from a selective action at H2-receptor. Since in addition to its blocking activity, ranitidine does not possess anti-and androgenic activity func-tion and also does not inhibit the mixed oxygenase metabolizing enzyme system in the liver (Feldman et al., 1998),it possesses a long duration of action (El – Serag ans Sonnenberg, 1998).
Conclusion
From the results obtained in this study, lime juice significantly enhanced gastric acid secretion and therefore may not be beneficial to peptic ulcer patients. Also, results obtained shows that there is significantly higher gastric secretion in females than in males. This means that peptic ulcer may be predominant in females than in male. Again, there was more secretion in rats with higher weight than in those with lower weight; this implies that peptic ulcer patients should be conscious of weight gain. Also, it was observed that insulin caused hyperacid-ity (hypoglycemia), therefore starvation should be avoided especially in peptic ulcer patients and since lime juiced caused a significant increase in gastric secretion, peptic ulcer patients should stay away from lime juice and derivatives. Finally, since Ranitidine produced a significant decrease in both lime juice and insulin-induced gastric secretion, it could pose a potent drug for peptic ulcer treatment.
References
- Aihara T, Nakamura E, Amagese k, Tomita K, Fujishita T, Furutani K, et al. Pharmacological control of gastric acid secretion for the treatment of acid-related pep-tic diseases, past, present and future. Pharmacol Ther. Apr.2003;98(1):109-27.
- Bonfils S, Mignon M, Roze C. Vagal control of gastric secretion: In InternationalReviewofPhysiology. Gas -trointestinalPhysiology. University Park Press, Baltimore, 1979; 59-106.
- Clayden J, Greevs N, Stuart k, Wothers P. Organic Chemistry, Oxford University Press.2001; 1: 204-6, 586-8.
- CodeCF, Blackbuon GR, Livernwre K. A method of quantitative determination of gastric secretory inhibition. Gastroenterology. 1949; 68: 573-88.
- Davenport HW. Gastric Carbonic anhydrase. JPhysiol. 1939; 8: 32-4.
- El-Serag HB, Sonnenberg A. Opposing time trends of peptic ulcer and reflux disease. Gut. 1998; 43 (3): 327-33.
- Feldman M, Scharschmidt BF, Sleisenger MH.Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. Pathophysiology, Diagnosis and Management. 6th ed. Philadelphia: W.B. Saunders Company. 1998; 48: 809-62.
- Forte JG, Zhu L. Apical Vecydiny of the gastric pariental cell H/K – Atpase. Annu Rev Physiol. 2010; 12: 273-96.
- Geibel JP. Role of potassium in gastric acid secre-tion. World J. Gastroenterol. 2005; 11: 5259.
- Hulma AC. The Biochemistry of fruits and their products. Academic Press London, New York. 1971; 2: 60-101, 485-505.
- Kokwaro J. O. Medicinal plants of East Africa. Uni-versity of Nairobi press, Kenya. 3rd Ed.2009; 1: 1-19.
- Konturek SJ, Taslor J, Cieszowski M, Dobranzan -ska M, Wunsch E. Stimulation of gastrin release and gas -tric secretion by amino acids bathing pyloric gland area. Am J Physiol. 1977; 233: 170-4.
- Lee FY, Leung KL, Lai BS, NG SS, Dexter S, Lau WY. Predicting mortality and morbidity of patients oper-ated on for perforated peptic ulcers. Arch Surg. 2001; 136: 90–94.
- OliverB. Medicinal plants in Nigeria. Nigeria col-lege of Art and Science and Technology, Ibadan. 1960; 7:33-138.
- Prema S, Smbulingam K. Essentials of Medical Physiology. JayPee publishers. 5th Ed. 2010; 5: 221-8. ISBN 9788184487046
- Prichard PJ, Jones DB, Yeomans ND, Mihaly GW, Smallwood RA, Louis WJ. The effectiveness of ranitidine in reducing gastric acid-secretion, decreases with contin -ued therapy. Br J Clin Pharmacol. Dec 1986;22(6):663-8.
- Reimer C, Sondergaard B, Hilsted L, Byper P. Pro-ton-pump inhibitor therapy induces acid related symptoms in healthy volunteers after withdrawal of therapy. Gastro-enterology. 2009; 137: 8.
- Roekpe TK, Anantharam A, Kirchhoff P, Busque SM, Young JB, Geibel JP et al. The KCNE2 Potas-sium channel ancillary subunit is essential for gastric acid secretion.Journal of Biological Chemistry.2006; 281, 23740-23747.
- Samuelson LC, Hinkle KL. Insight into the regula -tion of gastric acid secretion through analysis of geneti -cally engineered mice. Annu Rev Physiol. 2003; 65:383.
- Sandrik AK, Waldum HL. Aspect of the regulation of gastric Histamine release. Gastroenterology. 1991; 180: 108.
- Schubert,M.L, Peura DA. Control of gastric acid secretion in health and diseases. Gastroenterology. 2008; 134: 1842.
- Sembulingam K, Sembulingam P. Essentials of Medical Physiology. 2010; 56 -99.ISBN-10: 8184487045.
- Shulke A, Read M. Regulation of Somatostatin by gastrin and acid dependent mechanisms. Endocrinology. 1991; 129: 2329.
- Soll AH. Postaglandin inhibition of histamine stimulated aminopyrine uptake and cyclic AMP generation by isolated canine pariental cells. Gastroenterology. 1978; 74: 1146.
- Soll AH, Berglindh T. Receptors regulating acid secretory function. Physiology of the gastrointestinal tract. Raven Press, New York. 1994; 3: 1139.
- Soll A, Read M. Regulation of somatostatin secretion by gastrin and acid dependent mechanism. Endocrinology. 1991; 129: 2329.
- Trivett TL, Meyer EA. Citrate cycle and related metabolism of Listeria monocytogenes. J Bacte -riol. Sep 1971;107(3):770-9.
- Troidl H, LorenzW, Rohle H, Hafner G, Ronzhei-mer M, Schannl A. Histamine content in human gastric mucosa: its relation to pentagastrin-stimulated acid se -cretion and to selective-gastric vagotomy with drainage. Agents Actions. Dec 1975;5(5):427-8.
- Uvans B. Gastrin and vagus. Gastroenterology. 1969; 56: 812.
- Vasudevan D, Sreekumari S. Textbook of Bio-chemistry for medical student (JayPee). 2007; 5: 496.
- Ward S, Gilespie FL, Passaro BP, Grossman NI. Comparison of histamine and histology as stimulants for maximum gastric secretion in human subjects and in dogs. Gastroenterology.1963; 44: 620-6
- Wormsley KG. Pentagastrin Snuff – a new means of stimulating gastric secretion. lancet. Jan 131968; 1(7533):57-8.
- Yang H. Central and peripheral regulation of gas -tric acid by peptide YY. Peptides. Feb 2002; 23 (2):349-58.
- Yao X, Forte JG. Cell Biology of acid secretion by the parental cells. Annu Rev Physiol.2003;65:103-31. Epub 2002.