Study of the Correlation Between Bronchial Hyperresponsiveness and Exhaled Nitric Oxide in Subjects with Suspected Symptoms of Asthma
Van-Quang T1, Do-Van D2, Tran-Van H3, Duong-Quy S4,5,6*
1 Department of Health, Binh Duong Province, Vietnam.
2 Department of Health, Ninh Binh Province, Vietnam.
3 Department of Health Science, Thang Long University, Hanoi, Vietnam.
4 Department of Pulmonology and Lung Functional Testing, Cochin Hospital, Paris Descartes University, France.
5 Department of Immuno-Allergology, Penn State College of Medicine, USA.
6 Biomedical Research Center, Lam Dong Medical College, Dalat, Vietnam.
*Corresponding Author
Sy Duong-Quy MD, PhD, FCCP,
Professor, Cochin Hospital, Paris Descartes University Penn State Medical College, USA.
Director of Lam Dong Medical College and Biomedical Research Center.
E-mail: sduongquy.jfvp@gmail.com
Received: July 10, 2017; Accepted: July 28, 2017; Published: July 29, 2017
Citation: Van-Quang T, Do-Van D, Tran-Van H, Duong-Quy S (2017) Study of the Correlation Between Bronchial Hyper Responsiveness and Exhaled Nitric Oxide in Subjects with Suspected Symptoms of Asthma. Int J Clin Med Allergy. 5(2), 65-70. doi: dx.doi.org/10.19070/2332-2799-1700012
Copyright: Duong-Quy S© 2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Bronchial hyperresponsiveness (BHR) is one of main features of asthma within chronic inflammation and reversible bronchoconstriction. Actually, methacholine challenge is useful method to detect BHR in subjects with suspected asthma symptoms. However, this method has some limitations due to its safety and side effects. The measure of exhaled nitric oxide (NO) demonstrates currently as the alternative method for methacholine challenge.
Methods: Ninety-five subjects had at least one of the following symptoms were included in this study: wheezing or chest tightness during exercise, chronic cough, or nocturnal coughing. They were divided into two groups depending on the positivity or negativity of BHR. Lung function test, exhaled NO measurement, and methacholine challenge were done for each study subject.
Results: There were no significant differences between two groups for age and male/female ratio (41 ± 22 vs 38 ± 23 years old and 0.9 vs 1.1; P > 0.05 and P > 0.05; respectively). The percentage of wheezing and nocturnal coughing in subjects with positive BHR (BHR+) was significantly higher than that in subjects with negative BHR (BHR-: 70.9% and 64.5% vs 31.2% and 45.1%; P<0.001 and P<0.01; respectively). FENO measured at 50 mL/s in subjects with BHR+ was significantly higher subjects with BHR- (36 ± 10 ppb vs 11 ± 9 ppb; P<0.001). There was a significant correlation between FENO-50 mL/s and methacholine dose in subjects with BHR+ (R= -0.695; P<0.001). FENO-50 mL/s at 35 ppb had 86.7% of sensibility and 82.9% of specificity for diagnosis of BHR.
Conclusion: FENO is a useful biomarker for diagnosis of asthma in subjects with suspected symptoms of asthma. FENO level has a high sensitivity and specificity for screening out subjects with BHR. The measurement of exhaled NO may be an alternative method for detecting BHR in diagnosis of asthma in clinical practice.
1.Introduction
2.Objectives
3.Subjects and Methods
3.1 Study Subjects
3.2 Laboratory Techniques
4.Results
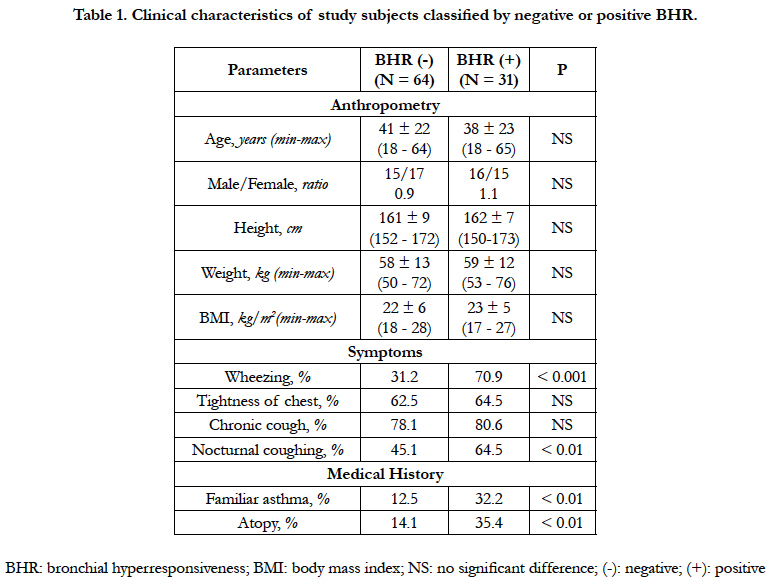
4.1 Clinical Characteristics of Study Subjects Classified by Negative or Positive BHR
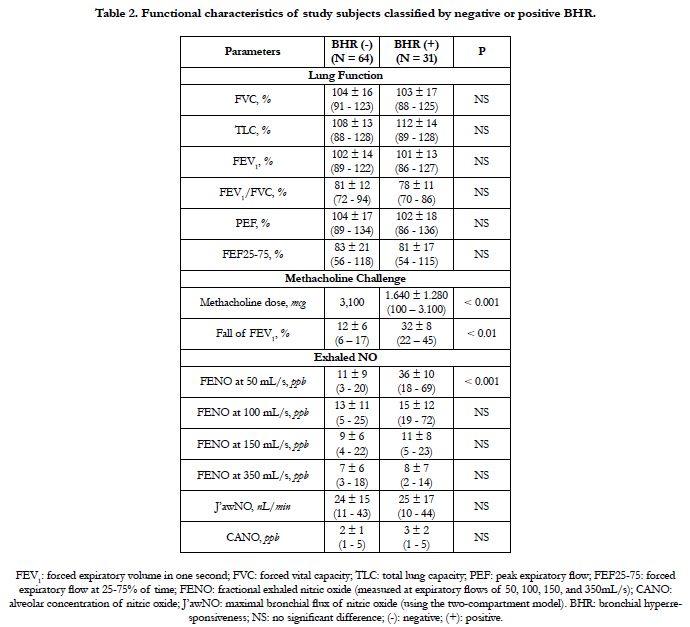
4.2 Functional Characteristics of Study Subjects Classified by Negative or Positive BHR
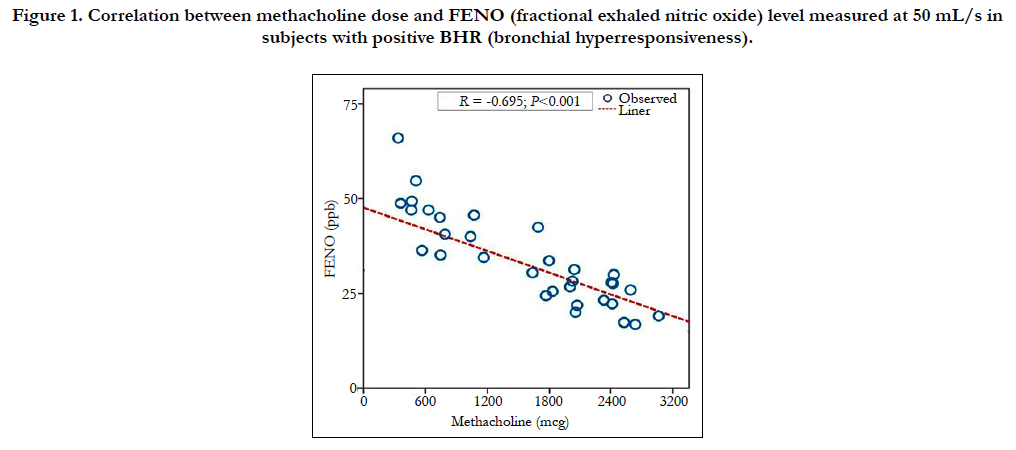
4.3 Correlation between Methacholine Dose and FENO Level in Subjects with Positive BHR
4.4 Cute-off of FENO Level for Screening Subjects with Positive BHR
5.Discussion
6.Conclusion
7.References
Keywords
Bronchial Hyperresponsiveness; Exhaled NO; Asthma; FENO; Methacholine.
Introduction
Bronchial hyperresponsiveness (BHR) is one of pathophysiological features usually associated with chronic inflammation and reversible obstruction of airways in patients with asthma [1]. This phenomenon is also found out in the pathogenesis of chronic obstructive pulmonary disease (COPD) and known as a risk factor for the development of respiratory symptoms as well as a predictor for lung functional impairment [2]. Clinically, BHR is defined by excessive bronchospasm responding to a specific or nonspecific stimulus. The use of methacholine to produce bronchoconstriction is the most common method for assessing the increase of bronchial responsiveness [3]. Methacholine is a chemical product that acts directly on smooth muscle cells producing bronchial constriction. The test of methacholine challenge is based on an association of increasing inhaled concentrations of methacholine and a repetitive measurements of FEV1 (forced expiratory volume in one second) with the drop of FEV1 more than 20%.
Methacholine challenge is useful for diagnosis of asthma [4], especially in patients without clinical symptom of asthma or without abnormalities in lung function measured by spirometry or whole body plethysmography. However, until now, the use of methacholine challenge to find out BHR for diagnosis of asthma is still not available in some medical centers, even though in some national hospital of respiratory diseases in developing countries. Moreover, methacholine challenge may produce severe bronchospasm in some patients and should be avoided in some clinical situations such as pregnant women or patients with severe cardiovascular diseases.
Recently, exhaled nitric oxide (NO) has been used as a biomarker of asthma because the concentration of NO in exhaled breath is significantly correlated with the level of inflammation in the airways [5]. The measure of NO in exhaled breath (FENO) is a non-invasive method for evaluating an allergic inflammation and plays an important role in the diagnosis and control of asthma [5-7]. In asthma, although FENO correlates significantly with other biomarkers of inflammation such as eosinophil count in sputum or total IgE in serum, the correlation between FENO and BHR is still controversial. It is due to the contradictory correlation between the level of bronchial FENO and the dose of methacholine to reduce 20% of FEV1 (PD20 - methacholine). Some authors found there was a significant correlation, while the others described no significant association [8-10]. These conflicting results might be explained by certain differences in study population or FENO measurement method.
Objectives
This study was planned to describe: 1) Level of exhaled NO in subjects with bronchial hyperresponsiveness (BHR) diagnosed by methacholine challenge; 2.) Correlation between the level of exhaled NO and the dose of methacholine in subjects with positive BHR. 3) Cute-off of exhaled NO for diagnosing BHR in study population.
It was a cross-sectional and descriptive study. Subjects who had been realized a methacholine challenge for diagnosis of BHR at Clinical Research Unit of Lam Dong Medical College were included in the study after signing an Institutional Review Boardapproved consent form. These subjects had one of following symptoms with suggestive asthma were included in the study: chronic or nocturnal cough, wheezing or chest tightness at night or during physical exercise.
Subjects who were active smokers in the last 5 years or used any inhaled corticosteroids (ICS) or systemic corticosteroids for less than 3 months were excluded from the study. All bronchodilators had been interrupted for at least 24 hours before all the tests. Subjects had any contraindication for methacholine challenge such as cardiovascular diseases, seizures, pregnancy, uncontrolled high blood pressure, or recent respiratory infection were excluded from the study. FENO measurement was made routinely before methacholine challenge testing.
LFT was done by using wholebody phlethysmography with Body Box 500 (Medisoft, Sorinnes, Belgium). The Body Box had been calibrated every day by using a standard three liter pump. Each study subject beneficed three best flow - volume curves for FEV1 and all values were selected in accordance with the ATS (American Thoracic Society)/ERS (European Respiratory Society) recommendations [11]. The data was presented as percentage of theoretical normal values.
Exhaled NO was measured at multiple flow rates (50 mL/s, 100 mL/s, 150 mL/s, and 350 mL/s) before methacholine challenge by using an electrochemical based analyzer FeNO+ (Medisoft, Sorinnes, Belgium). Technical measurement of exhaled NO was conducted according to manufacturer’s instructions, as recommended by the ATS/ERS guideline [6]. The maximal bronchial production rate of NO (J’awNO) and alveolar concentration of NO (CANO) were automatically determined using the two-compartment model and had been reported via Expair’s software (Medisoft) as described previously [12, 13].
Methacholine (acetyl methylcholine chloride) was used in the form of 5% concentration solution. Prior to each test, 2.5% solution of methacholine was prepared by diluting a stock solution in 2 mL of saline buffer (NaCl 0.9%). Study subject was in sitting posture, using nose clip and oral inhalation from an aerosol spray device integrated in Body Box 500 and it was automatically activated by computer software. After initial measurement with methacholine - free diluted saline, inhalation of methacholine through multiple steps at double dose steps was starting at 100 mcg up to a maximum cumulative dose of 3,100 mcg.
FEV1 value was measured at 60 seconds after each step and the lowest value was recorded. The methacholine challenge had been stopped when FEV1 dropped more than > 20% of baseline or after reaching the maximum dose. All study subjects were given systematically 400 mcg of Ventolin inhalation after methacholine challenge and remained in the waiting room until their FEV1 values returned to above 90% of the initial values. The accumulated dose of methacholine causing 20% reduction of FEV1 (PD20 - methacholine) was calculated from linear equation of response curve - dose. BHR was positive when there was a decrease of FEV1 ≥ 20% with methacholine dose ≤ 3,100 mcg.
Statistical analysis was performed by using SPSS software (version 22.0; Chicago IL; USA). Values were expressed as mean ± standard deviation for quantitative variables and percentage for qualitative variables. The comparison between groups was done by Student’s t-test. The Pearson coefficient was used to evaluate the correlation between parameters and statistical significance as determined by P value < 0.05. The ROC curve was used to evaluate the predictive value of FENO for diagnosis of BHR.
Ninety-five subjects were included in this study. They were divided into two groups: 64 subjects with negative BHR and 31 others with positive BHR. There were no significant differences between two groups for age and male/female ratio (41 ± 22 vs 38 ± 23 years old and 0.9 vs 1.1; P > 0.05 and P > 0.05; respectively; Table 1). The body mass index (BMI) did not differ between two groups (22 ± 6 vs 23 ± 5; P > 0.05). Concerning suspected symptoms of asthma, there were no significant differences between two groups for the percentage of tightness of chest and chronic cough (62.5% and 78.1% vs 64.5% and 80.6%; P > 0.05 and P > 0.05; Table 1).
The percentage of wheezing and nocturnal coughing in subjects with positive BHR was significantly higher than that in subjects with negative BHR (70.9% and 64.5% vs 31.2% and 45.1%; P < 0.001 and P < 0.01; respectively; Table 1). In subjects with positive BHR, the percentage of subjects had a familiar asthma or an atopy was higher than that in those with negative BHR (P < 0.01 and P < 0.01; respectively; Table 1).
The results of present study showed that there were no significant differences between two groups for lung function parameters (Table 2). The mean percentage of FEV1 and FEV1/FVC ratio was not significantly different between subjects with negative or positive BHR (102 ± 14% and 81 ± 12% vs 101 ± 13% and 78 ± 11%; P > 0.05 and P > 0.05; respectively; Table 2). The dose of methacholine using for provocation test to diagnosis negative or positive BHR was significantly different between two groups (3,100 mcg for negative BHR vs 1,640 mcg for positive BHR; P < 0.001). The fall of FEV1 in subjects with positive BHR was significantly higher than that for those with negative BHR (32 ± 8% vs 12 ± 6%; P < 0.01; Table 2).
The result of exhaled NO measurement showed that the levels of FENO measured at 100 mL/s - 150 mL/s - 350 mL/s were not significant differences between subjects with negative BHR and positive BHR (P > 0.05, P > 0.05, and P > 0.05; respectively; Table 2). The level of FENO measured at 50 mL/s in subjects with positive BHR was significantly higher than that in subjects with negative BHR (36 ± 10 ppb vs 11 ± 9 ppb; P < 0.001; Table 2). There was no significant difference between CANO levels between positive and negative BHR (3 ± 2 ppb vs 2 ± 1 ppb; P > 0.05; Table 2).
The linear regression equation between the dose of methacholine and the level (concentration) of FENO with the flow of 50 mL/s (bronchial level) in subjects with positive BHR showed that there was a significant correlation between these two parameters (Figure 1). This correlation was negative and strong (R = - 0.695; P < 0.001; Figure 1). The multivariate regression analysis showed the correlation between methacholine dose and FENO was independent with anthropometric parameters (age, gender, height or weight, and BMI; data not showed) and lung function parameters (FEV1, FVC, and TLC; data not showed).
Figure 1. Correlation between methacholine dose and FENO (fractional exhaled nitric oxide) level measured at 50 mL/s in subjects with positive BHR (bronchial hyperresponsiveness).
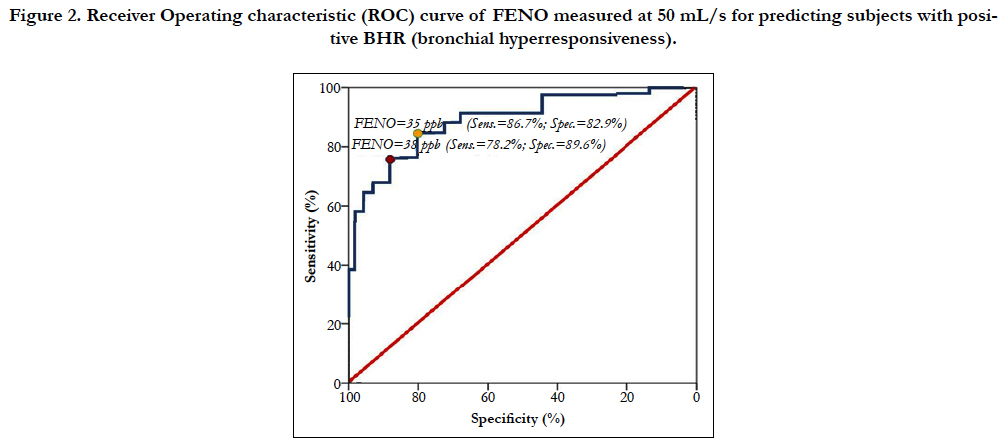
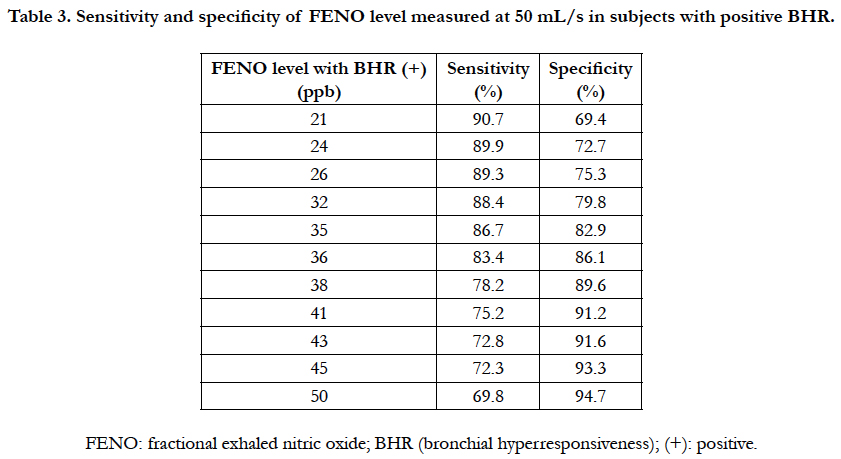
The receiver operating characteristic (ROC) curve of FENO level measured at 50 mL/s in subjects with positive BHR was performed to measure the sensitivity and specificity of different levels of FENO for screening positive BHR (Figure 2). The level of FENO at 35 ppb had a highest Youden’s Index (or highest area under curve) with 86.7% of sensibility and 82.9% of specificity (Figure 2 and Table 3). With the level of FENO at 38 ppb, the sensitivity and specificity was 78.2% and 89.6%, respectively (Figure 2 and Table 3).
Figure 2. Receiver Operating characteristic (ROC) curve of FENO measured at 50 mL/s for predicting subjects with positive BHR (bronchial hyperresponsiveness).
Table 3. Sensitivity and specificity of FENO level measured at 50 mL/s in subjects with positive BHR.
Discussion
Currently, the relationship between FENO and BHR identified by methacholine challenge is still controversial. In this study, the correlation between the dose of methacholine causing significant bronchoconstriction (PD20 - methacholine) and the concentration of NO in exhaled breath (FENO) measured at 50 mL/s has been demonstrated (Figure 1). Especially, FENO level of subjects with positive BHR (BHR+) was significantly higher than that for those with negative BHR (BHR-; Table 2). In addition, subjects with BHR (+) had the percentages of wheezing, nocturnal coughing, familiar asthma, and atopy were significantly higher than subjects with BHR (-). It suggests that these clinical symptoms might be used as markers of BHR.
In subjects with asthma, BHR is often present and considered as one of the features of this disease. In asthma, FENO is also increased and similar with chronic airways inflammation and associated with hypereosinophilic. According to current recommendations, FENO can be used to diagnose and monitor the treatment of asthma [14, 15]. In patients with atypical symptoms of asthma, presented by chronic cough, wheezing, nocturnal coughing, or dyspnea during exertion, methacholine challenge is a relevant and helpful tool to determine the persistence of non-specific bronchial responsiveness and then to confirm the diagnosis of asthma. The present study suggests that FENO level measured at 50 mL/s may be used as a biomarker for diagnosis of BHR. FENO measured at 50 mL/s (FENO - 50 mL/s) is used currently to evaluate the bronchial inflammation in subjects with asthma [6, 15, 16]. In adult, the normal level of FENO measured at 50 mL/s is lower than 25 ppb. The result of our study showed that FENO – 50 mL/s ≥ 35 ppb can be used to diagnose BHR because of its high sensitivity and high specificity (Figure 2 and Table 2). However, when using the high level of FENO, there was a significant decrease of sensitivity and increase of specificity. Inversely, the sensitivity of FENO level to diagnose BHR was decreased when the level of FENO between 25 - 35 ppb (Table 3).
Measuring exhaled NO is easier to realize than methacholine challenge testing. This technique is safer than methacholine challenge to determine BHR. Actually, the use of portable of FENO measurement is expanded in many countries. FENO is also a good biomarker to diagnose asthma and to follow-up asthma control [17-20]. Whereas, methacholine is a chemical product and not available in some areas and countries with low resources. Moreover, the result of our study suggests that in some situations and in subjects with contraindications of methacholine challenge such pregnant women or subjects with severe cardiovascular diseases, the measure of FENO may be an alternative method to screen out BHR. Finally, methacholine challenge does not seem to be necessary to be done in subjects with high level of FENO (positive prediction) or low level of FENO (negative prediction). However, more studies on the correlation between FENO and BHR in subjects with atypical symptoms of asthma should be realized in the future to clarify the role of FENO in diagnosis of BHR [21].
Conclusion
Bronchial hyperresponsiveness (BHR) is one of asthma features. The present of BHR is useful for clinicians to diagnose asthma in subjects with atypical symptoms. However, the use of methacholine challenge to confirm BHR is not available in some respiratory centers and unsafe in some clinical settings. Hence, the measure of FENO is a relevant alternative method to screen out BHR in subjects with suspected symptoms of asthma.
References
- Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM (2000) Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 161(5): 1720–1745.
- O’Connor G, Sparrow D, Weiss S (1995) A prospective longitudinal study of methacholine airway responsiveness as a predictor of pulmonary-function decline: the Normative Aging Study. Am J Respir Crit Care Med. 152(1): 87–92.
- Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, et al., (2000) Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American thoracic society was adopted by the Ats board of directors, july 1999. Am J Respir Crit Care Med. 161(1): 309-29.
- N Scichilone, M Messina, S Battaglia, F Catalano, V Bellia (2005) Airway hyperresponsiveness in the elderly: prevalence and clinical implications. Eur Respir J. 25(2): 364-375.
- Kharitonov SA, Barnes PJ (2001) Exhaled markers of pulmonary disease. Am J Respir Crit Care Med. 163(7): 1693-722.
- American Thoracic Society, European Respiratory Society (2005) ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 171(8): 912–930.
- Dinh-Xuan AT, Annesi-Maesano I, Berger P, Chambellan A, Chanez P, et al., (2015) Contribution of exhaled nitric oxide measurement in airway inflammation assessment in asthma. A position paper from the French Speaking Respiratory Society. Rev Mal Respir. 32(2): 193–215.
- A Jatakanon, S Lim, SA Kharitonov, KF Chung, PJ Barnes (1998) Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax. 53(2): 91–95 91.
- Delclaux C, Zerah-Lancner F, Mahut B, Ribeil S, Dubois A, et al., (2005) Alveolar nitric oxide and effect of deep inspiration during methacholine challenge. Chest. 127(5): 1696-702.
- Berkman N, Avital A, Breuer R, Bardach E, Springer C, et al., (2005) Exhaled nitric oxide in the diagnosis of asthma: comparison with bronchial provocation tests. Thorax. 60(5): 383-8.
- Miller MR, Crapo R, Hankinson J, Burgos F, Coates A, et al., (2005) General considerations for lung function testing. Eur Respir J. 26(1): 153-61.
- Duong-Quy S, Hua-Huy T, Tran-Mai-Thi HT, Le-Dong NN, Craig TJ, et al., (2016) Study of exhaled nitric oxide in subjects with suspected obstructive sleep apnea: a pilot study in Vietnam. 2016: 3050918.
- NM Tsoukia, SC George (1998) A two-compartment model of pulmonary nitric oxide exchange dynamics. J Appl Physiol. 85(2): 653–666.
- Guo Z, Wang Y, Xing G, Wang X (2016) Diagnostic accuracy of fractional exhaled nitric oxide in asthma: a systematic review and meta-analysis of prospective studies. J Asthma. 53(4): 404-412.
- Global Initiative for Asthma (GINA), 2015.
- Nguyen-Thi-Bich H, Duong-Thi-Ly H, Thom VT, Dinh LD, Craig TJ, et al., (2016) Study of the correlations between fractional exhaled nitric oxide in exhaled breath and atopic status, blood eosinophils, FCER2 mutation, and asthma control in Vietnamese children. J Asthma Allergy. 9: 163-170.
- Smith AD, Cowan JO, Filsell S, Jackson P, Taylor DR, et al., (2004) Diagnosing asthma: comparisons between exhaled nitric oxide measurements and conventional tests. Am J Respir Crit Care Med. 169(4): 473-478.
- Cockcroft DW, Davis BE (2006) Mechanisms of airway hyperresponsiveness. J Allergy Clin Immunol. 118(3): 551-9.
- Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR (2005) Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med. 352(21): 2163–2173.
- Duong-Quy S, Hua-Huy T, Doan-Quynh N, Nguyen-Quoc B, Le-quang K, et al., (2015) A study of exhaled NO (FENO) measurement used to determine asthma control, dose of inhaled corticosteroid and cost in a developing country. Eur Respir J. 46(59): PA5013.
- Kim YH, Sol IS, Yoon SH, Kim MJ, Kim KW, et al., (2017) Association of extended nitric oxide parameters with bronchial hyperresponsiveness and bronchodilator response in children with asthma. J Breath Res. doi: 10.1088/1752-7163/aa7c1f.
- Park YA, Park HB, Kim YH, Sul IS, Kim HR, et al., (2017) Airway hyperresponsiveness to mannitol and methacholine and exhaled nitric oxide in children with asthma. J Asthma. 5: 1-8. doi: 10.1080/02770903.2016.1255751.
- Lee JW, Shim JY, Kwon JW, Kim HY, Lee SY, et al., (2015) Exhaled nitric oxide as a better diagnostic indicator for evaluating wheeze and airway hyperresponsiveness in preschool children. J Asthma. 52(10): 1054-9.