Thyroid Dysfunction in Libyan Vitiligo Patients
El-Dibany SA1*, El-Sherif NA2, SH Greiw A3, Matmati NA4, Belkhair N2
1 Dermatology Department, Omar El-Mukhtar University, Al-Beida, Libya.
2 Faculty of Medicine, Department of Dermatology and Venereology, Benghazi University, Libya.
3 Faculty of Medicine, Department of Family and Community Medicine, Benghazi University, Libya.
4 Radiology Department, 7 October hospital, Benghazi, Libya.
*Corresponding Author
Salwa A. El-Dibany,
Dermatology Department,
Omar El-Mukhtar university, Al-Beida, Libya.
Email: salwaeldibani@yahoo.com
Received: March 03, 2017; Accepted: April 21, 2017; Published: April 27, 2017
Citation: El-Dibany SA, El-Sherif NA, SH Greiw A, Matmati NA, Belkhair N (2017) Thyroid Dysfunction in Libyan Vitiligo Patients. Int J Clin Dermatol Res, S2:002, 4-7. doi: dx.doi.org/10.19070/2332-2977-SI02002
Copyright: El-Dibany SA© 2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Vitiligo is an acquired depigmenting disorder due to destruction of melanocytes. Many theories have been suggested for its pathogenesis. One of these theories showed that there is evidence that autoimmunity and endocrine dysfunction may be involved. The association of vitiligo with autoimmune thyroid diseases and the increased prevalence of autoantibodies including thyroid autoantibodies in vitiligo favor this role.
Aim of the study: was to determine whether vitiligo is associated with thyroid autoimmunity.
Patients and Methods: Fifty Libyan patients with vitiligo and 50 controls matched by age and sex were enrolled. Patients were excluded if they had a history of thyroid, or other autoimmune diseases. Data on age, onset of illness, duration and disease activity were determined. Serum T3, T4, TSH, and antibodies to TPO and TG were measured in both vitiligo patients & controls. All patients and control subjects underwent thyroid ultrasonography.
Results: Fifty patients with vitiligo and their 50 matched controls were studied. More than half of the patients (52%) were females and 48% were males, their mean of age was 40 ± 11 years, and the duration of vitiligo was 11± 9 years. Vitiligo vulgaris type was the most common form seen in 68% of the patients, and 42% reported at least one family member affected with vitiligo. Family history of thyroid disorder was seen in 20% of the patients. Thyroid functional abnormalities were significantly seen more in patients than control subjects. The frequency of TG and TPO thyroid autoantibodies was significantly higher in vitiligo patients than in healthy controls (P < 0.01). Abnormal thyroid ultrasound study was seen in 18 (36%) of the patients compared to 6 (12%) of the control subjects (P < 0.05).
Conclusion: Our findings pointed to a significant association between vitiligo and thyroid autoimmunity and showed that testing the level of thyroid autoantibodies is relevant in vitiligo patients.
2.Patients and Methods
2.1 Ethical Consideration
2.2 Laboratory Investigations
3.Statistical Analysis
4.Results
5.Discussion
6.Conclusion
7.References
Introduction
Vitiligo is the most common, probably heritable, progressive depigmenting skin disorder caused by destruction of melanocytes. It is characterized by well-demarcated white patches of skin. Depending on the extent of the lesions, vitiligo can be classified into two main categories: generalized and localized. It affects 0.5-2% of the general population with no age, sex, or racial predilection [1]. Many different etiologic hypotheses have been presented for vitiligo. The most recent of these hypotheses supports the combination of environmental and genetic factors interacting to contribute to autoimmune melanocytes destruction [1, 2]. Several studies reported that there is an epidemiological association between generalized vitiligo and other autoimmune diseases; such as autoimmune thyroid diseases, pernicious anemia, alopecia areata and Addison’s disease [3-5]. The aim of the study was to evaluate the association between vitiligo and thyroid autoimmune diseases.
Patients and Methods
Fifty Libyan patients with vitiligo and 50 healthy controls matched by age and sex were evaluated at the Dermatology Departments of Al Jamahiriya hospital at Benghazi city, Libya. The control subjects were recruited from healthcare workers. Patients and control subjects were excluded if they had a history of thyroid, or other autoimmune diseases. The diagnosis of vitiligo was mainly clinical. A detailed history including the age of onset, duration of the disease, localization of the lesions, disease activity and presence of Koebner phenomenon, associated diseases and family history were obtained from each patient.
The approval of the manager of the hospital was taken. Signed informed consent was obtained from each patient and control subjects.
Fasting blood samples were collected between 08:30 and 10:00 h, after an overnight fasting of more than 12 hours. The blood samples were analysed for total triiodothyronine (T3), total thyroxine (T4), thyroid stimulating hormone (TSH), thyroid peroxidase (TPO) antibody and thyroglobulin (TG) antibody. Thyroid gland sonography was performed for all patients and control subjects by the same radiologist.
Statistical Analysis
Data were fed to computer using Statistical Package for Social Sciences (SPPS) version 11.5 and reviewed. Descriptive statistics in the form of percentages mean and standard deviation of different parameters was used. Chi square test was used to compare qualitative parameters, P < 0.05.
Results
Fifty patients with vitiligo and their 50 matched controls were studied. Of the 50 vitiligo patients, 26 (52%) were females and 24 (48%) were males, with a mean age of 40 years and a mean age of onset of the disease ± SD was 21 ± 7 years. The mean duration of vitiligo ± SD was 19 ± 10 years (range: 1 - 45 years).
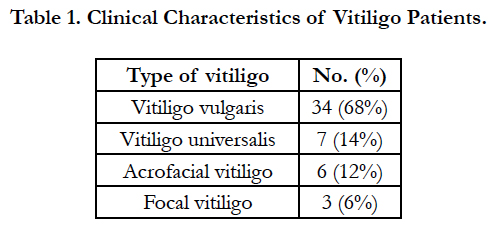
The distribution pattern of the lesions, denoting the clinical type of vitiligo, is shown in Table 1. Vitiligo vulgaris was the most common type, followed by universal, acrofacial, and focal types. Koebner phenomenon was observed in 26 (52%) of the patients.
Of the 50 cases, 21 (42%) had a family history of vitiligo, firstdegree relatives (parents⁄siblings) were affected in 18 (36%) of the patients and second degree relatives (grandparents, uncles or aunts) in 3 (6%) of the patients. Ten (20%) of the patients had family history of thyroid diseases. Six (12%) of the patients had family history of hypothyroidism, 2 (4%) had hyperthyroidism, and 2 (4%) had family history of goiter.
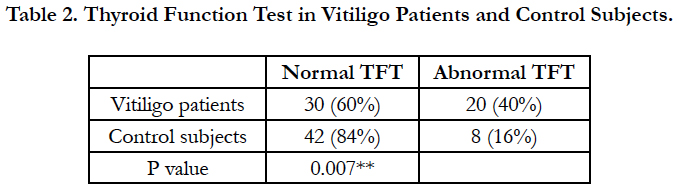
Thyroid functional abnormalities were seen in 20 (40%) of the patients compared to 8 (16%) of the control subjects and this difference was statistically significant (Table 2).
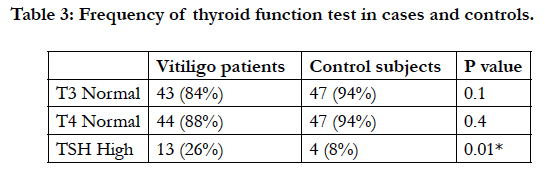
The T3 and T4 were within normal range in 42 (84%) and 44 (88%) of the patients, and 47 (94%) and 47 (94%) of control group, respectively, however, no significant difference was detected between the two groups (Table 3).
The frequency of high TSH was 26% in vitiligo patients and 8% in control subjects and this difference was statistically significant (P = 0.016) (Table 3). None of the patients or control subjects had low TSH level.
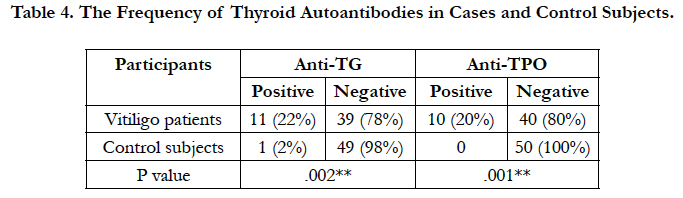
Anti-TG antibody in 11 (22%), and anti-TPO antibody in 10 (20%) of the patients were higher than the normal antibodies titer. Moreover, 5(10%) of the patients had both anti-TG and anti-TPO antibodies higher than the normal antibody titer. In the control group, one subject (2%) had positive anti-TG and none had positive anti-TPO (Table 4). This difference was statistically significant.
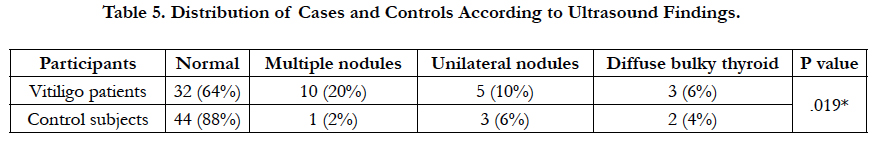
Abnormal thyroid ultrasound study was seen in 18 (36%) of the patients compared to 6 (12%) of the control subjects (P < 0.05) (Table 5).
Discussion
Vitiligo is an acquired pigmentary disorder of unknown etiology characterized by depigmented macules resulting from the loss of melanocytes. The prevalence of the disease ranges from 0.1% to 8% with female predominance [1]. The onset of the disease usually occurs before 20 years of age. Generalized vitiligo is the most common clinical presentation and often involves the face and acral regions. The course of the disease is unpredictable, with a severe psychological impact on the patient quality of life and the response to treatment varies [1].
The pathogenesis of vitiligo is complex and involves the interplay of a series of variables [5, 6]. There is a multifactorial genetic component predisposing certain individuals to vitiligo and family history is a variable found in approximately one-third of the people with the disease [7]. There is also strong genetic evidence of a link between vitiligo and other autoimmune diseases [8].
The autoimmune hypothesis of vitiligo proposes that an immune system disorder results in the destruction of melanocytes. The epidemiological association with several autoimmune diseases is one of the various evidences which support an autoimmune origin of vitiligo [2, 3]. Among these diseases, lichen planus, pernicious anemia, Hashimoto’s thyroiditis, hypothyroidism, endemic goiter, diabetes mellitus, lupus erythematosus, and Graves’ disease. However, the chance of coexistence with autoimmune thyroid diseases is higher than the others [9-12].
In 1941, Robert suggested that vitiligo might be connected with an increased activity of the thyroid gland; and reported a distinct rise of the basal metabolism in 10 out 20 vitiligo patients tested [3]. Later, several studies reported a significantly increased prevalence of autoimmune thyroid disease in vitiligo patients; the rate of positivity of thyroid autoantibodies varied from 2.2% to 50% [2, 4].
In the present study Anti-TG antibody was found in 22%, and anti-TPO antibody was found in 20% of the patients. Our results are consistent with previous studies [11, 13, 14]. A recent study of 87 vitiligo patients demonstrated positive anti-TG titers in 23% of the patients and anti-TPO antibody were positive in 24.1% of the patients, and the results were found significantly higher when compared to healthy controls [14]. Moreover, study of Kasumagic- Halilovic et al., found high levels of anti-TPO in 11 (27%) out of 40 vitiligo patients and they demonstrated significantly elevated levels of anti-TPO compared to the control group [11].
Daneshpazhooh et al., measured the serum level of anti-TPO antibody and reported significantly high levels in vitiligo patients compared to healthy controls [10]. Moreover, a study was carried out in India revealed that the anti-TPO antibody was positive in 31.4% cases [15].
However, Gulcan Saylam Kurtipek, et al., found high levels of anti-TPO in 16 (14.8%) and anti-TG in 9 (8.3%) out of 108 vitiligo patients, these rates are lower than our study [16]. In addition, previous studies reported a significantly increased prevalence of vitiligo in patients with autoimmune thyroid disease compared to patients with non-autoimmune thyroid disease [10, 16].
In present study, thyroid dysfunction manifesting as hypothyroidism occurred in 26% of the patients and in 8% of controls, this difference being statistically significant. A high prevalence of hypothyroidism in vitiligo patients has been reported by Kumar et al. ,(40%) and Akay et al., (31%) [17, 18]. However, a lower occurrence was noted in other study [19]. Although, previous study have noticed an association of vitiligo with hyperthyroidism, these findings were not noticed in our study [20].
Conclusion
The study revealed a significant association between vitiligo and thyroid autoimmunity and screening vitiligo patients for thyroid autoantibody seems plausible.
References
- Alikhan A, Felsten LM, Daly M, Petronic-Rosic V (2011) Vitiligo: A comprehensive overview Part I. Introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and workup. J Am Acad Dermatol. 65(3): 473–91.
- Al-Mutairi N, Sharma A (2006) Profile of vitiligo in Farwaniya region in Kuwait. Kuwait Med J. 38(2): 128–131.
- Robert P (1941) Ueber die vitiligo. Dermatology. 84: 317–319.
- Mandry RC, Ortíz LJ, Lugo-Somolinos A, Sánchez JL (1996) Organ-specific autoantibodies in vitiligo patients and their relatives. Int J Dermatol. 35(1): 18–21.
- Schallreuter KU, Bahadoran P, Picardo M, Slominski A, Elassiuty YE, et al., (2008) Vitiligo pathogenesis: autoimmune disease, genetic defect, excessive reactive oxygen species, calcium imbalance, or what else? Exp Dermatol. 17(2): 139–140.
- Richmond JM, Frisoli ML, Harris JE (2013) Innate immune mechanisms in vitiligo: danger from within, Current Opinion in Immunology. 25(6): 676–682.
- Faria AR, Mira MT, Tarl´e RG, Silva de Castro CC, Dellatorre G (2014) Vitiligo-part 2-classification, histopathology and treatment. An Bras de Dermatologia. 89(5): 784–790.
- Spritz RA (2013) Modern vitiligo genetics sheds new light on an ancient disease. J Dermatol. 40(5): 310–318.
- Niepomniszcze H, Amad RH (2001) Skin disorders and thyroid diseases. J Endocrinol Invest. 24(8): 628–638.
- Daneshpazhooh M, Mostofizadeh GM, Behjati J, Akhyani M, Robati RM (2006) Anti-thyroid peroxidase antibody and vitiligo: a controlled study. BMC Dermatology. 6: 3.
- Kasumagic-Halilovic E, Ovcina-Kurtovic N, Jukic T, Karamehic J, Begovic B, et al., (2013) Vitiligo and autoimmunity. Med Arhiv. 67(2): 91–93.
- Vrijman C, Kroon MW, Limpens J, Leeflang MM, Luiten MM, et al., (2012) The prevalence of thyroid disease in patients with vitiligo: a systematic review. Br J Dermatol. 167(6): 1224–1235.
- Sedighe M, Gholamhossein G (2008) Thyroid dysfunction and thyroid antibodies in Iranian patients with vitiligo. Indian J Dermatol. 53(1): 9–11.
- Yang Y, Huang G, Yan X, Qing Z (2014) Clinical analysis of thyroglobulin antibody and thyroid peroxidase antibody and their association with vitiligo. Indian J Dermatol. 59(4): 357–360.
- Dave S, Dsouza M, Thapp DM, Reddy KS, Bobby Z (2003) High frequency of thyroid dysfunction in Indian patients with vitiligo. Indian J Dermatol. 48(2): 68–72.
- SaylamKurtipek G, Cihan FG, EraymanDemirbas S, Ataseven A (2015) The Frequency of Autoimmune Thyroid Disease in Alopecia Areata and Vitiligo Patients. BioMed Res Int. ID : 435947.
- Kumar KV, Priya S, Sharma R, Kapoor U, Saini M, et al., (2012) Autoimmune thyroid disease in patients with vitiligo: Prevalence study in India. Endocr Pract. 18(2): 194–9.
- Akay BN, Bozkir M, Anadolu Y, Gullu S (2010) Epidemiology of vitiligo, associated autoimmune diseases and audiological abnormalities: Ankara study of 80 patients in Turkey. J Eur Acad Dermatol Venereol. 24(10): 1144–50.
- Narita T, Oiso N, Fukai K, Kabashima K, Kawada A, et al., (2011) Generalized vitiligo and a sociated autoimmune diseases in Japanese patients and their families. Allergol Int. 60(4): 505–8.
- Iacovelli P, Sinagra JL, Vidolin AP, Marenda S, Capitanio B, et al., (2005) Relevance of thyroiditis and of other autoimmune diseases in children with vitiligo. Dermatology. 210(1): 26–30.