Binary Logistic Regression Analysis Of Foramen Magnum Dimensions For Sex Determination In Nigerian Skulls
Chinna Nneka Orish*, Lekpa Kingdom David, Chinagorom Ibeachu. Josiah S Hart
Department of Anatomy. Faculty of Basic Medical Science, College of Health Sciences, University of Port-Harcourt, Rivers State, Nigeria.
*Corresponding Author
Chinna Nneka Orish,
Department of Anatomy. Faculty of Basic Medical Science, College of Health Sciences, University of Port-Harcourt, Rivers State, Nigeria.
E-mail: chinna.orish@uniport.edu.ng
Received: September 05, 2022; Accepted: September 22, 2022; Published: September 26, 2022
Citation: Chinna Nneka Orish, Lekpa Kingdom David, Chinagorom Ibeachu. Josiah S Hart. Binary Logistic Regression Analysis Of Foramen Magnum Dimensions For Sex Determination In Nigerian Skulls. Int J Anat Appl Physiol. 2022;8(1):211-217.
Copyright: Chinna Nneka Orish@2022. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: Identification of skeletal is a vital tool in forensic investigations. The need to estimate sex from cranial fragments
becomes apparent when only a part of the skull is brought for identification. The dimensions of the foramen magnum
are clinically important because of the vital structures passing through it. This study investigated the sexual dimorphism of
the sagittal diameter, transverse diameter and area of the foramen magnum inthe Nigerian population.
Materials and methods: The sagittal and transverse diameters and area of the foramen magnum of one hundred skulls (83
males and 17 females) from the Nigerian population were measured using Verniercalliperr. The SPSS was used to analyze
linear correlation, histogram, Q-Q plot, and Binary Logistic Regression (BLR) to obtain a model for sex determination. The
student’st-test was used to test male-female significance. The predicted probabilities of BLR were analyzed using Receiver
Operating Characteristic (ROC) curve.
Result: The sagittal diameter, transverse diameter and area of the foramen magnum are found to be significantly larger in
males than in females. The predictability of foramen magnum measurements in sexing of crania was 83.3% for transverse
diameter and 92.3% for the sagittal diameter. For the area of foramen magnum that was calculated using the formula derived
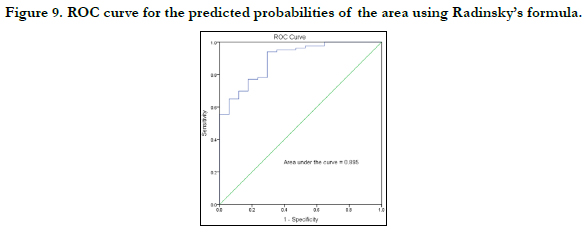
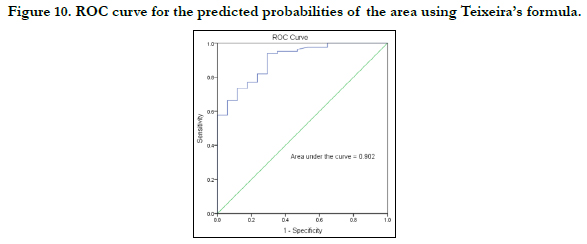
by Radinsky and Teixeira, the predicted probabilities were 89.5% and 90.2% respectively.
Conclusion: This study shows that there is statistically significant sexual dimorphism in the foramen magnum region, which
will be useful in predicting sex in partial skull remains by binary function analysis when other methods tend to be inconclusive.
This is an invaluable tool in forensic identification.
2.Introduction
3.Conclusion
4.References
Keywords
Foramen Magnum; Sex Determination; Binary Logistic Regression; Anthropometry.
Introduction
The foramen magnum is the most conspicuous, large opening on
the floor of the fossa. It transmits the medulla, the ascending portions
of the spinal accessory nerve (XI), and the vertebral arteries.
Configuration and size of the foramen magnum and posterior
fossa (PF) play an important role in the pathophysiology of various
disorders of the PF and craniovertebral junction. It is however
of high clinical importance as reported by various researchers.
Stenosis of the foramen magnum causes brainstem compression
manifested by respiratory complications, lower cranial nerve dysfunctions,
upper and lower extremity paresis, hypo-or hypertonia,
hyperreflexia, or clonus % [1-3]. The configuration of the foramen
magnum in patients with Chiari I and Chiari II malformations
is larger than in the normal population [4].
In forensic investigations, one of the principal biological traits to
be estimated from skeletal remains is the sex of the individual.
Sex estimation relies on the sexually dimorphic expression of
bony characteristics that are produced through different patterns,
rates and periods of adolescent growth [5]. Sex can be determined
by a gross examination of the skeleton using morphometric techniques.
In man, sex estimation using the human cranium is largely
based on size differences and skeletal robusticity [5, 6]. Several
workers have determined sex with reasonable accuracy using various
cranial landmarks and morphometries [7-10]. The observed differences in sex predictions using bones are thought to be population-
specific and can be influenced by genetic, environmental
and social factors [11].
This study has employed binary linearregression BLR analysisin
predicting sexual dimorphism of the sagittal, transverse diameters
and area of the foramen magnum in a Nigerian population to
evaluate the reliabilityof the foramen magnum morphometry as a
tool for sexing when only a fragment of base of the skull is available
in forensic identification.
Materials and Methods
A total of 100 adult dry human skulls, free from damage and
deformity fully ossified (83 males, 17 females) from Departments
of Anatomy in Nigerian Universities constituted the materials for
this study. The sagittal and transverse diametersof the foramen
magnum were measured.
The index and area of foramen magnum were calculated using the following formulas:
(a) Radinsky’s formula: = 1/4 × p × s + t
(b) Teixeira’s formula: = p× {(s+t )/4}2.
(c) Foramen magnum index = (transverse length)/(Sagittal×100).
Statistical analysis was performed using SPSS (Statistical Package
for Social Sciences, version 20.0) two-tailed Student’s -test
(< 0.05), Receiver Operating Characteristic (ROC) curve, Quantile-
Quantile plot, linear correlation, Binary Logistic Regression
(BLR), Binary Logistic Regression is applied to obtain a predicting
equation (BLR model) that estimates the sex of the individual.
An equation is obtained for each variable and on applying the
equation to the variable value apredicted value is obtained. In this
model, the cut-off value is 0.5. If the predicted value is equal to
or more than 0.5, it is considered male and if it is less than 0.5 it
is considered to be female. The predicted probabilities of BLR
were analyzed using Receiver Operating Characteristic (ROC)
curve. The ROC curve is a strong indicator of the model’s ability
to distinguish two groups and the area under the curve is used to
measure the strength of the equation. If the area is less than 0.5,
it indicates that any observation is purely a matter of chance and a
value close to 1 indicates that the equation strongly discriminates
two groups.
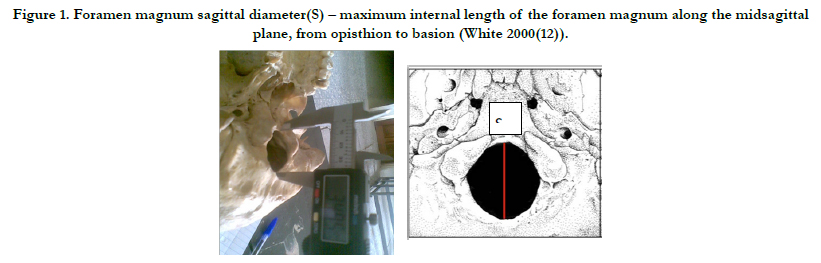
Figure 1. Foramen magnum sagittal diameter(S) – maximum internal length of the foramen magnum along the midsagittal plane, from opisthion to basion (White 2000(12)).
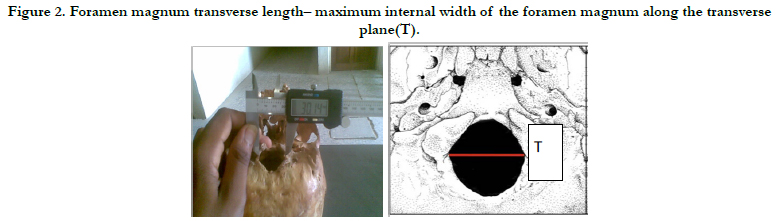
Figure 2. Foramen magnum transverse length– maximum internal width of the foramen magnum along the transverse plane(T).
Results
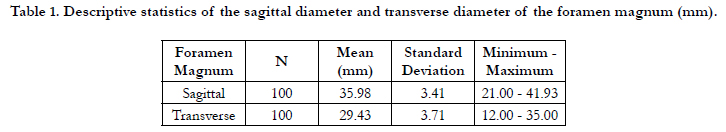
Table 1 shows the descriptive statistics of the sagittal diameter
and transverse diameter of the foramen magnum (mm). The
mean of the sagittal and transverse diameters were 35.98±3.41
and 29.43 ± 3.71mm respectively. The differences between these
values were statistically significant (< 0.001) and were consistent
with the shape of the foramen.
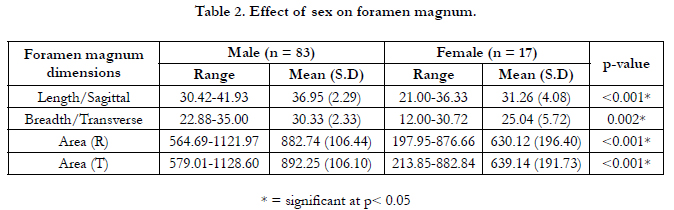
Table 2: The effect of sex on the foramen magnum is shown in
Table 2. The mean sagittal diameter in males was 36.95 ± 2.29
mm and in females was 31.26 ± 4.08 mm. The mean transverse
diameter in males was 30.33 ± 2.33 mm and in females was 25.04
± 5.72 mm. The mean sagittal area by Radinsky’s formula in males
was 882.74 ± 106.44 mm and in females was 630.12 ± 196.4 mm.
The mean transverse area by Teixeira’s formula in males was
892.25 ± 106.10 mm and in females was 639.14 ± 191.73 mm.
The differences were statistically significant (p< 0.05).
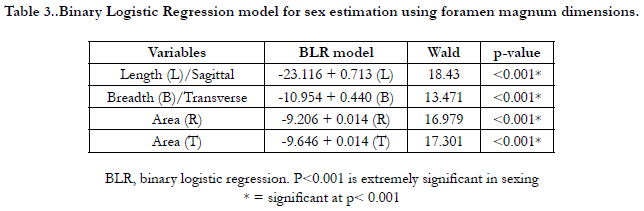
The BLR model for each variable is shown in Table 3 and on
applying the model any predicted value <0.5 is considered to be
female and equal to or more than 0.5 as male.
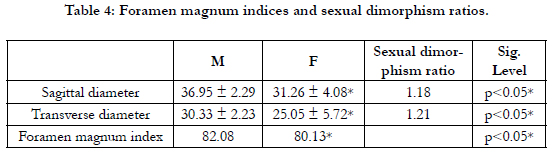
Table 4 shows the foramen magnum indices and sexual dimorphism
ratio. The foramen magnum indices in males and females
were 82.08 and 80.13 respectively with higher values in males.
The sexual dimorphism ratio using the Sagittal diameter was 1.18
whereas the sexual dimorphism ratio using the Transverse diameter
was 1.21. The sexual dimorphism ratios were greater than
the unity.
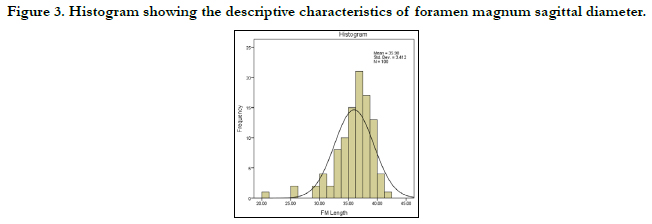
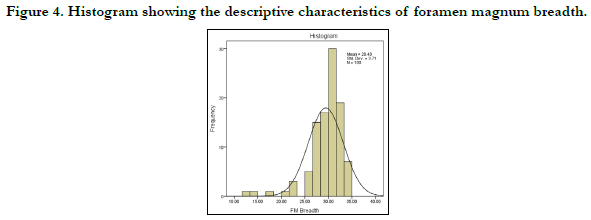
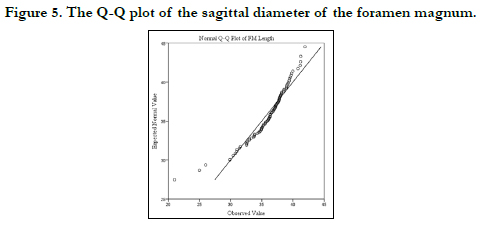
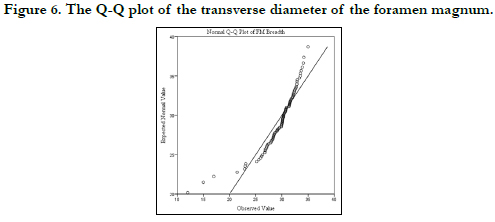
Figures 3 and 4 show the histograms of the descriptive characteristics of foramen magnum sagittal diameter and breadth/transverse respectively. High interindividual variability was seen in the sagittal and transverse diameter in Figures 3 and 4 respectively. The Q-Q plot of the sagittal and transverse diameters of the foramen magnum are shown in Figures 5 and 6 respectively. The Q-Q plots confirm normal distribution.
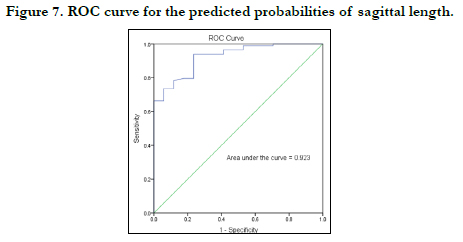
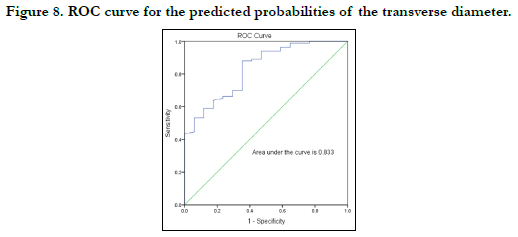
The strength of each model was then tested by the area under the Receiver Operating Characteristic (ROC) curve drawn for the predicted probabilities of BLR. The ROC curve of each variable is shown in Figures 7-10. The area under the curve is a measure of the predictability of the variable in sexing the crania. The area under the curve was 0.923 and 0.833mm2 for sagittal and transverse diameters respectively. The area of foramen measured by Radinsky’s method was 0.895 and 0.902 for the area of foramen measured by Teixeira’s method.
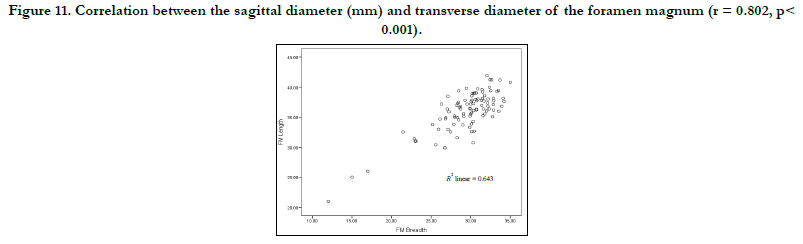
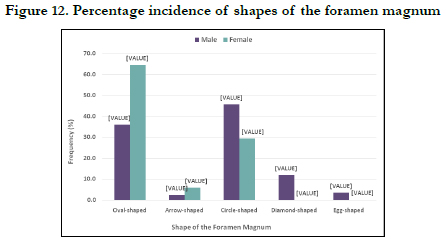
The Correlation between the sagittal and transverse diameters of the foramen magnum is shown in Figure 11. There is a strong positive linear correlation between the sex-pooled sagittal and transverse diameters of the foramen (value = 0.802) and the correlation are significant (< 0.001) as shown in Figure 10.Figure 12 shows the incidence of male and female foramen magnum shapes. The majority of the skulls in males were circle-shaped (45.8%), while 64.7% of females were oval-shaped. The males had 36.1% and females 64.7% oval-shaped, males 2.4% and females 5.9% arrow-shaped, males 12 and 3.6% diamond and eggshaped respectively without having any of these shapes.
Figure 11. Correlation between the sagittal diameter (mm) and transverse diameter of the foramen magnum (r = 0.802, p< 0.001).
Discussion
It is generally accepted that in cases where a human skull shows
better preservation than the pelvis, the basicranial and specifically
the area around the foramen magnum can be used for sexing an
unknown individual. It is especially relevant for highly fragmented
remains when the cranial base preserves well due to the large mass
of soft tissue and ligaments surrounding it [10, 13].
In this present study, the mean dimensions of the foramen were
higher in males than in females. This agrees with previous studies
[13-21]. The present study has employed descriptive statistics, and
predictive models and applied Binary Logistic Regression. The
differences in the data may be attributed to ethnicity and sample
size. The present study confirms the sex predictability employing
any of these parameters. It is observed that the sex predictability
is highest for sagittal diameter (92.3%), followed by the area
of Teixeira (90.2% and Radinsky (89.9%) and least for transverse
diameter (83.3%) which agree with the work done by Babu and
coworkers [17].
Kamath et al 2015 [21]. however, reported the sex predictability
is highest for bothareas 70.3%, followed by sagittal diameter
(69.6%), and least for transverse diameter (66.4%). In another
study using 118 dry skulls in a south Indian ethnic group, it was
observed that the areas of the foramen calculated by Radinsky’s
and Teixeira’s formulae are better predictors of sex than the sagittal
and transverse dimensions [22].
Gapert and coworkers in a British ethnic group study using discriminant
function and regression analysis predicted a sexing accuracy
of 70.3% [20]. In the analysis of CT scans of 250 adults
from the Swiss ethnic group to determine the value of foramen
magnum dimensions in sexing crania, revealed 66% accuracy in
cranial sexing by discriminant function analysis and Binary Logistic
Regression% [23]. In another study, involving fifty adult skulls,
the accuracy of sex prediction based on discriminant function
analysis ranged from 66% to 70% [24].
Several workers have concluded that the foramen exhibits sexual
dimorphism [25-30] used Fisher’s linear discriminant function test
on three-dimensional computed tomography measurements and
concluded 81% accuracy in sexing with foramen width and right
condyle dimension [29]. used helical CT scanning in the measurements
of the foramen diameters, area, and circumference
was statistically analyzed using discriminant analysis and multiple
regression analysis to suggest that circumference and area were
the best discriminant parameters for sex determination with an
overall accuracy of 67% and 69.3%. The present study however
has turned out higher predictable values of 90.2 and 89.5% using
Teixeira and Radinsky models respectively.
Foramen magnum indices in the present study are 82.03 and 80.13
in males and femalesrespectively which is inconsistent with the
work done by Madadin et al 2017 [30]. However, another study
has reported higher values in females than males [31]. The significant
positive correlation between length and breadth of foramen
observed in the present study agrees with previous studies [21,
32] Our study also shows that the sagittal diameter was significantly
larger than the transverse diameter. This observation is in
agreement with reports using skulls from other ethnic regions [21,
33-35].
Usually, the morphology of the foramen magnum is classified by
visual assessment into seven shape types. A Kenyan based study
involving two hundred and two adult skulls recorded that the
shape of the foramen magnum was oval, circular, and polygonal
in 13%, 24%, and 63% of the cases, respectively [36]. This present
study reported the majority of the skull in females tobe ovalshaped
(64.7% while a majorityof the males were circle-shaped
(45.8%) which conforms with reports by other researchers [32].
Taken together, the present study lends further credence to the
use of foramen sexing of skulls in forensic science since morphometric
considerations of the foramen are important for neurosurgeons,
radiologists and anthropologists. Although foramen
magnum dimensions appear to demonstrate statistically significant
differences between the sexes, singular or isolated use of this
method is not encouraged unless as a suggestive finding when
other features of assessment are absent or limited.
References
- Bagley CA, Pindrik JA, Bookland MJ, Camara-Quintana JQ, Carson BS. Cervicomedullary decompression for foramen magnum stenosis in achondroplasia. J Neurosurg. 2006 Mar;104(3 Suppl):166-72. PubMed PMID: 16572633.,/
- Dickman CA, Spetzler RF, Sonntag VK, editors. Surgery of the craniovertebral junction. New York: Thieme; 1998 Jun.
- Wang Y, Xiao R, Yang F, Karim BO, Iacovelli AJ, Cai J, et al. Abnormalities in cartilage and bone development in the Apert syndrome FGFR2(+/ S252W) mouse. Development. 2005 Aug;132(15):3537-48. PubMed PMID: 15975938.
- Gardner WJ, Goodall RJ. The surgical treatment of Arnold-Chiari malformation in adults; an explanation of its mechanism and importance of encephalography in diagnosis. J Neurosurg. 1950 May;7(3):199-206. PubMed PMID: 15415776.
- Rogers TL. Determining the sex of human remains through cranial morphology. J Forensic Sci. 2005 May;50(3):493-500. PubMed PMID: 15932077.
- Hamilton ME. Sexual dimorphism in skeletal samples. Sexual dimorphism in Homo sapiens: a question of size. 1982:107-63.
- Giles E, Elliot O. Sex determination by discriminant function analysis of crania. Am J Phys Anthropol. 1963 Mar;21(1):53-68. PubMed PMID: 13947858.
- Franklin D, Freedman L, Milne N. Sexual dimorphism and discriminant function sexing in indigenous South African crania. Homo. 2005;55(3):213- 28. PubMed PMID: 15803767.
- FrancesquiniJúnior L, Francesquini MA, De La Cruz BM, Pereira SD, Ambrosano GM, Barbosa CM, et al. Identification of sex using cranial base measurements. J Forensic Odontostomatol. 2007 Jun;25(1):7-11. PubMed PMID: 17577972.
- Saini V, Srivastava R, Shamal SN, Singh TB, Kumar V, Kumar P, et al. Temporal variations in basicranium dimorphism of North Indians. Int J Legal Med. 2014 Jul;128(4):699-707. PubMed PMID: 24374986.
- Rösing FW, Graw M, Marré B, Ritz-Timme S, Rothschild MA, Rötzscher K, et al. Recommendations for the forensic diagnosis of sex and age from skeletons. Homo. 2007;58(1):75-89. PubMed PMID: 17306261.
- White TD, Black MT, Folkens PA. Human osteology. Academic press; 2011 Jan 21.
- Gruber P, Henneberg M, Böni T, Rühli FJ. Variability of human foramen magnum size. Anat Rec (Hoboken). 2009 Nov;292(11):1713-9. PubMed PMID: 19777568.
- Olivier G. Biometry of the human occipital bone. J Anat. 1975 Dec;120(Pt 3):507-18. PubMed PMID: 1213952.
- Routal RR, Pal GP, Bhagwat SS, Tamankar BP. Metrical studies with sexual dimorphism in foramen magnum of human crania. J AnatSoc India. 1984;33(2):85-9.
- Sayee R, Janakiram S, Thomas IM. Foramen magnum measurements of Crania from Karnataka. J AnatSoc India. 1987;36(2):87-9.
- RaghavendraBabu YP, Kanchan T, Attiku Y, Dixit PN, Kotian MS. Sex estimation from foramen magnum dimensions in an Indian population. J Forensic Leg Med. 2012 Apr;19(3):162-7. PubMed PMID: 22391003.
- Murshed KA, Çiçekcibasi AE, Tuncer I. Morphometric evaluation of the foramen magnum and variations in its shape: a study on computerized tomographic images of normal adults. Turk J Med Sci. 2003;33(5):301-6.
- Catalina-Herrera CJ. Study of the anatomic metric values of the foramen magnum and its relation to sex. ActaAnat (Basel). 1987;130(4):344-7. Pub- Med PMID: 3434189.
- Gapert R, Black S, Last J. Sex determination from the foramen magnum: discriminant function analysis in an eighteenth and nineteenth century British sample. Int J Legal Med. 2009 Jan;123(1):25-33. PubMed PMID: 18553095.
- Kamath V, Asif M, Shetty R, Avadhani R. Binary logistic regression analysis of hard palate dimensions for sexing human crania. Anat Cell Biol. 2016 Jun;49(2):151-9. PubMed PMID: 27382518.
- Kanchan T, Gupta A, Krishan K. Craniometric analysis of foramen magnum for estimation of sex. Int J Med Health Biomed Pharm Eng. 2013 Jul 29;7:111-3.
- Edwards K, Viner MD, Schweitzer W, Thali MJ. Sex determination from the foramen magnum. Journal of forensic radiology and imaging. 2013 Oct 1;1(4):186-92.
- Singh G, Talwar I. Morphometric analysis of foramen magnum in human skull for sex determination. Human biology review. 2013;2(1):29-41.
- Catalina-Herrera CJ. Study of the anatomic metric values of the foramen magnum and its relation to sex. ActaAnat (Basel). 1987;130(4):344-7. Pub- Med PMID: 3434189.
- Holland TD. Use of the cranial base in the identification of fire victims. J Forensic Sci. 1989 Mar;34(2):458-60. PubMed PMID: 2708959.
- Uysal S, Gokharman D, Kacar M, Tuncbilek I, Kosa U. Estimation of sex by 3D CT measurements of the foramen magnum. J Forensic Sci. 2005 Nov;50(6):1310-4. PubMed PMID: 16382824.
- Ukoha U, Egwu OA, Okafor IJ, Anyabolu AE, Ndukwe GU, Okpala I. Sexual dimorphism in the foramen magnum of Nigerian adult. Int J Biol Med Res. 2011;2(4):878-81.
- Uthman AT, Al-Rawi NH, Al-Timimi JF. Evaluation of foramen magnum in gender determination using helical CT scanning. DentomaxillofacRadiol. 2012 Mar;41(3):197-202. PubMed PMID: 22116135.
- Madadin M, Menezes RG, Al Saif HS, Abu Alola H, Al Muhanna A, Gullenpet AH, et al. Morphometric evaluation of the foramen magnum for sex determination: A study from Saudi Arabia. J Forensic Leg Med. 2017 Feb;46:66-71. PubMed PMID: 28157592.
- Kumar A, Dave M, Anwar S. Morphometric evaluation of foramen magnum in dry human skulls. Int J Anat Res. 2015;3(2):1015-23.
- Shanthi CH, Lokanadham S. Morphometric study on foramen magnum of human skulls. Med Sci. 2013;2(4):792-8.
- Kanodia G, Parihar V, Yadav YR, Bhatele PR, Sharma D. Morphometric analysis of posterior fossa and foramen magnum. J Neurosci Rural Pract. 2012 Sep;3(3):261-6. PubMed PMID: 23188974.
- Shepur MP, Magi M, Nanjundappa B, Havaldar PP, Gogi P, Saheb SH. Morphometric analysis of foramen magnum. Int J Anat Res. 2014;2(1):249-55.
- Patel R, Mehta CD. Morphometric study of Foramen Magnum at the base of human skull in South Gujarat. IOSR-JDMS. 2014 Jun;13(6):23-5.
- Loyal P, Ongeti K, Pulei A, Mandela P, Ogeng’o J. Gender related patterns in the shape and dimensions of the foramen magnum in an adult Kenyan population. Anatomy Journal of Africa. 2013;2(2):138-41.